Why do you believe a profit motive is necessary to end our addiction to fossil fuels?
See my previous post
There's no shortage of empirical evidence proving human-generated CO2 is causing the earth to heat up:
Great...lets see some empirical evidence proving that human generated CO2 is causing the earth to heat up. This may surprise you, but to date, there has not been a single peer reviewed paper in which the warming that we are supposed to be causing has been empirically measured, quantified, and blamed on our so called greenhouse gasses...since no such paper exists, exactly where does all this evidence "proving" that we are causing the earth to warm up reside...don't you think there would be at least one published paper on the topic if such evidence existed?
"Atmospheric CO2 levels (Green is
Law Dome ice core, Blue is
Mauna Loa, Hawaii) and Cumulative CO2 emissions (
CDIAC). While atmospheric CO2 levels are usually expressed in parts per million, here they are displayed as the amount of CO2 residing in the atmosphere in gigatonnes. CO2 emissions includes fossil fuel emissions, cement production and emissions from gas flaring.
Sorry guy...your graph is nothing more than an exercise in curve fitting..it doesn't show anything like our year to year emissions...it has all been smoothed in order to create an impression....if you want to see our actual CO2 emissions vs the amount of CO2 in the atmosphere year to year, look at the graphs I provided above which are all from peer reviewed, published literature
"The final piece of evidence is ‘the smoking gun’, the proof that CO2 is causing the increases in temperature.
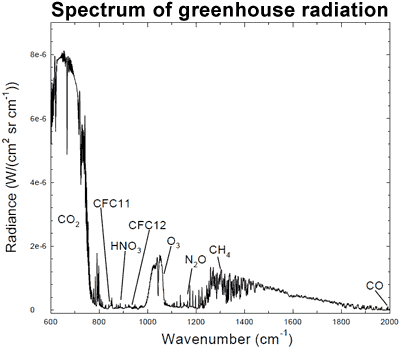
"CO2 traps energy at very specific wavelengths, while other greenhouse gases trap different wavelengths. In physics, these wavelengths can be measured using a technique called spectroscopy. Here’s an example:
"Spectrum of the greenhouse radiation measured at the surface. Greenhouse effect from water vapor is filtered out, showing the contributions of other greenhouse gases (Evans 2006).
Sorry guy, but that is not evidence that CO2 is causing anything...that is nothing more than an absorption spectrum which shows pretty clearly that CO2 only absorbs in a very small portion of the infrared spectrum emitted by earth..and it only shows half the picture...that shows the absorption spectrum...there is an emission spectrum which goes with that which shows that all that radiation absorbed by all the so called greenhouse gasses is immediately emitted...nothing is held back, nothing is blocked, nothing is trapped..it is absorbed and emitted...
Now there is one gas that can absorb and retain energy, but your graph doesn't show it...wonder why? That gas would be H2O...or water vapor...and your pseudoscientific graph leaves it out because it completely dominates the infrared spectrum rendering CO2 impotent...and unlike the rest of the so called greenhouse gasses, H2O can actually retain the energy it absorbs.

"The graph shows different wavelengths of energy, measured at the Earth’s surface. Among the spikes you can see energy being radiated back to Earth by ozone (O3), methane (CH4), and nitrous oxide (N20).
"But the spike for CO2 on the left dwarfs all the other greenhouse gases, and tells us something very important: most of the energy being trapped in the atmosphere corresponds exactly to the wavelength of energy captured by CO2."
As you can see, H2O completely dominates CO2...and again, your graph is an absorption spectrum...it isn't an emission spectrum...an emission spectrum would show that all the energy being absorbed by CO2 is then immediately emitted on to space...although, CO2 generally doesn't actually get to emit any radiation at all.
But that isn't really the entire story either...the fact is that it is estimated that about 8% of the energy emitted from the surface of the earth actually radiates through the troposphere...the rest is moved via convection and conduction...which means that a radiative greenhouse effect as described by climate science simply is not possible...which explains why you are unable to provide any empirical evidence of it. Here is an email exchange between Dr William Happer...you may have heard of him...he is a physicist who resides on the very top shelf of scientists in his field, which is atomic physics, optics, and spectrometry...
Dear Prof. Happer,
At your UNC lecture you told us many things which I had not known, but two of them were these:
1. At low altitudes, the mean time between molecular collisions, through which an excited CO2 molecule can transfer its energy to another gas molecule (usually N2) is on the order of 1 nanosecond.
2. The mean decay time for an excited CO2 molecule to emit an IR photon is on the order of 1 second (a billion times as long).
Did I understand that correctly? [YES, PRECISELY. I ATTACH A PAPER ON RADIATIVE LIFETIMES OF CO2 FROM THE CO2 LASER COMMUNITY. YOU SHOULD LOOK AT THE BENDING-MODE TRANSITIONS, FOR EXAMPLE, 010 – 000. AS I THINK I MAY HAVE INDICATED ON SLIDE 24, THE RADIATIVE DECAY RATES FOR THE BENDING MODE ALSO DEPEND ON VIBRATION AND ROTATIONAL QUANTUM NUMBERS, AND THEY CAN BE A FEW ORDERS OF MAGNITUDE SLOWER THAN 1 S^{-1} FOR HIGHER EXCITED STATES. THIS IS BECAUSE OF SMALL MATRIX ELEMENTS FOR THE TRANSITION MOMENTS.]
You didn't mention it, but I assume H2O molecules have a similar decay time to emit an IR photon. Is that right, too? [YES. I CAN'T IMMEDIATELY FIND A SIMILAR PAPER TO THE ONE I ATTACHED ABOUT CO2, BUT THESE TRANSITIONS HAVE BEEN CAREFULLY STUDIED IN CONNECTION WITH INTERSTELLAR MASERS. I ATTACH SOME NICE VIEWGRAPHS THAT SUMMARIZE THE ISSUES, A FEW OF WHICH TOUCH ON H2O, ONE OF THE IMPORTANT INTERSTELLAR MOLECULES. ALAS, THE SLIDES DO NOT INCLUDE A TABLE OF LIFETIMES. BUT YOU SHOULD BE ABLE TO TRACK THEM DOWN FROM REFERENCES ON THE VIEWGRAPHS IF YOU LIKE. ROUGHLY SPEAKING, THE RADIATIVE LIFETIMES OF ELECTRIC DIPOLE MOMENTS SCALE AS THE CUBE OF THE WAVELENTH AND INVERSELY AS THE SQUARE OF THE ELECTRIC DIPOLE MATRIX ELEMENT (FROM BASIC QUANTUM MECHANICS) SO IF AN ATOM HAS A RADIATIVE LIFETIME OF 16 NSEC AT A WAVELENGTH OF 0.6 MIRONS (SODIUM), A CO2 BENDING MODE TRANSITION, WITH A WAVELENGTH OF 15 MICRONS AND ABOUT 1/30 THE MATRIX ELEMENT SHOULD HAVE A LIFETIME OF ORDER 16 (30)^2 (15/.6)^3 NS = 0.2 S.
So, after a CO2 (or H2O) molecule absorbs a 15 micron IR photon, about 99.9999999% of the time it will give up its energy by collision with another gas molecule, not by re-emission of another photon. Is that true (assuming that I counted the right number of nines)? [YES, ABSOLUTELY.]
In other words, the very widely repeated description of GHG molecules absorbing infrared photons and then re-emitting them in random directions is only correct for about one absorbed photon in a billion. True? [YES, IT IS THIS EXTREME SLOWNESS OF RADIATIVE DECAY RATES THAT ALLOWS THE CO2 MOLECULES IN THE ATMOSPHERE TO HAVE VERY NEARLY THE SAME VIBRATION-ROTATION TEMPERATURE OF THE LOCAL AIR MOLECULES.]
Here's an example from the NSF, with a lovely animated picture, which even illustrates the correct vibrational mode:
Carbon Dioxide Absorbs and Re-emits Infrared Radiation | UCAR Center for Science Education
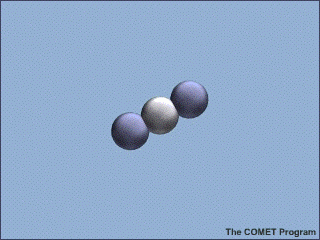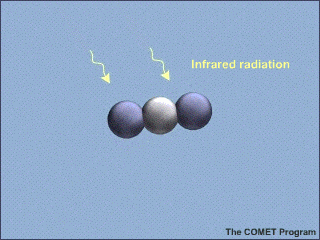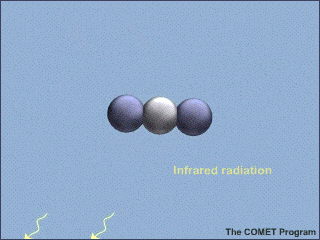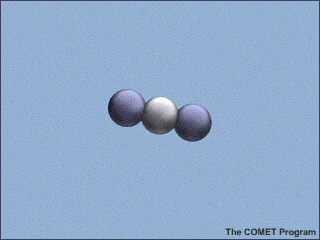
Am I correct in thinking that illustration is wrong for about 99.9999999% of the photons which CO2 absorbs in the lower troposphere? [YES, THE PICTURE IS A BIT MISLEADING. IF THE CO2 MOLECULE IN AIR ABSORBS A RESONANT PHOTON, IT IS MUCH MORE LIKELY ( ON THE ORDER OF A BILLION TIMES MORE LIKELY) TO HEAT THE SURROUNDING AIR MOLECULES WITH THE ENERGY IT ACQUIRED FROM THE ABSORBED PHOTON, THAN TO RERADIATE A PHOTON AT THE SAME OR SOME DIFFERENT FREQUENCY. IF THE CO2 MOLECULE COULD RADIATE COMPLETELY WITH NO COLLISIONAL INTERRUPTIONS, THE LENGTH OF THE RADIATIVE PULSE WOULD BE THE DISTANCE LIGHT CAN TRAVEL IN THE RADIATIVE LIFETIME. SO THE PULSE IN THE NSF FIGURE SHOULD BE 300,000 KM LONG, FROM THE EARTH'S SURFACE TO WELL BEYOND A SATELLITE IN GEOSYNCHRONOUS ORBIT. THE RADIATED PULSE SHOULD CONTAIN 667 CM^{-1} *3 X 10^{10} CM S^{-1}*1 S WAVES OR ABOUT 2 TRILLION WAVES, NOT JUST A FEW AS IN THE FIGURE. A BIT OF POETIC LICENSE IS OK. I CERTAINLY PLEAD GUILTY TO USING SOME ON MY VIEWGRAPHS. BUT WE SHOULD NOT MAKE TRILLION-DOLLAR ECONOMIC DECISIONS WITHOUT MORE QUANTITATIVE CONSIDERATION OF THE PHYSICS.]
(Aside: it doesn't really shock me that the NSF is wrong -- I previously caught them contradicting Archimedes:
before &
after.)
If that NSF web page & illustration were right, then the amount of IR emitted by CO2 or H2O vapor in the atmosphere would depend heavily on how much IR it received and absorbed. If more IR was emitted from the ground, then more IR would be re-emitted by the CO2 and H2O molecules, back toward the ground. But I think that must be wrong.[YES, THE AMOUNT OF RADIATION EMITTED BY GREENHOUSE MOLECULES DEPENDS ALMOST ENTIRELY ON THEIR TEMPERATURE. THE PERTRUBATION BY RADIATION COMING FROM THE GROUND OR OUTER SPACE IS NEGLIGIBLE. CO2 LASER BUILDERS GO OUT OF THEIR WAY WITH CUNNING DISCHARE PHYSICS TO GET THE CO2 MOLECULES OUT OF THERMAL EQUILIBRIUM SO THEY CAN AMPLIFY RADIATION.]
If 99.9999999% of the IR absorbed by atmospheric CO2 is converted by molecular collisions into heat, that seems to imply that the amount of ~15 micron IR emitted by atmospheric CO2 depends only on the atmosphere's temperature (and CO2 partial pressure), not on how the air got to that temperature. [YES, I COULD HAVE SAVED A COMMENT BY READING FURTHER.] Whether the ground is very cold and emits little IR, or very warm and emits lots of IR, will not affect the amount of IR emitted by the CO2 in the adjacent atmosphere (except by affecting the temperature of that air). Is that correct? [YES, PRECISELY. WE HAVE BEEN TALKING ABOUT WHAT CHANDRASEKHAR CALLS AN “ABSORBING ATMOSPHERE” AS OPPOSED TO A “SCATTERING ATMOSPHERE.” ASTROPHYSICISTS ARE OFTEN MORE INTERESTED IN SCATTERING ATMOSPHERES, LIKE THE INTERIOR OF THE SUN. THE BLUE SKY DURING A CLEAR DAY IS AN EXAMPLE OF SCATTERING ATMOSPHERE. VERY LITTLE HEATING OR COOLING OF THE AIR OCCURS WITH THIS “RAYLEIGH SCATTERING.”]
Thank you for educating a dumb old computer scientist like me! [YOU ARE HARDLY DUMB. YOU GET AN A+ FOR THIS RECITATION SESSION ON RADIATIVE TRANSFER. ]
You provide a link to skeptical science and call it evidence...are you kidding? Do tell..which part of that do you believe to be empirical evidence that humans are causing global warming...feel free to cut and paste.
"Summing Up
Like a detective story, first you need a victim, in this case the planet Earth: more energy is remaining in the atmosphere.
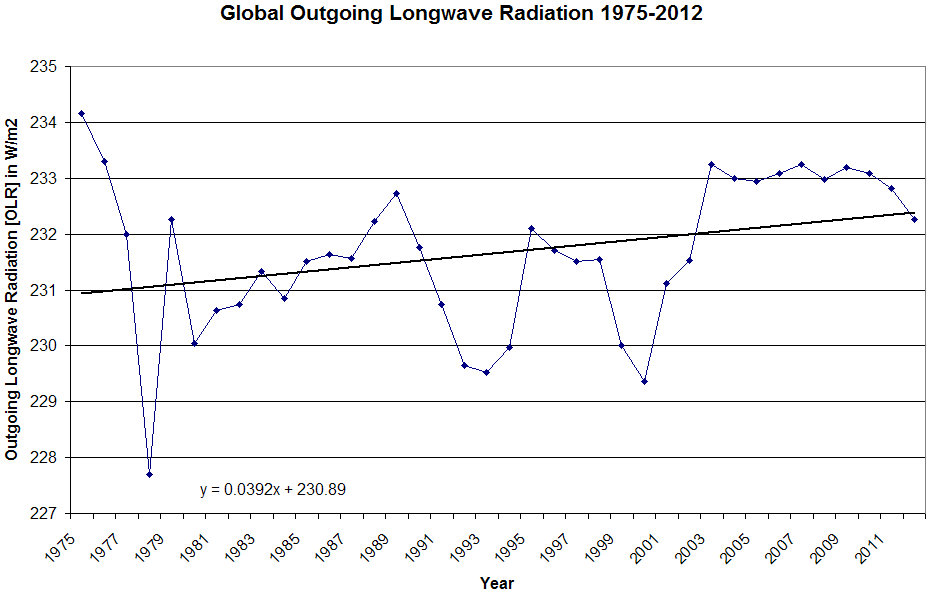
That isn't what the observation shows...observation shows that more energy is escaping the earth at the top of the atmosphere as the amount of greenhouse gasses increases..Your hypothesis fails right out of the gate...you have been lied to and tricked. Why is that so hard to admit when the observed, measured evidence proves it beyond question?
Then you need a method, and ask how the energy could be made to remain. For that, you need a provable mechanism by which energy can be trapped in the atmosphere, and greenhouse gases provide that mechanism.
Since the observations show us that energy is not being trapped, and in fact the amount of energy leaving the earth is increasing, your point is meaningless...your mechanism is non existent...the argument is based on a lie...
Next, you need a ‘motive’. Why has this happened? Because CO2 has increased by nearly 50% in the last 150 years and the increase is from burning fossil fuels.
Again..the observations show precisely the opposite of what you are claiming...outgoing radiation is not decreasing...it is increasing..and has been for a good long time.
And finally, the smoking gun, the evidence that proves ‘whodunit’: energy being trapped in the atmosphere corresponds exactly to the wavelengths of energy captured by CO2.
Brilliant deduction...except it is completely wrong...again...the observations show that the amount of energy exiting the earth that the top of the atmosphere is increasing...and there is no upper tropospheric hot spot which would be inevitable if energy were being trapped by so called greenhouse gasses...your case is built on assumptions which observation proves to be wrong..
The last point is what places CO2 at the scene of the crime.
Yet another pointless point...Since your whole case is based on flawed information...there is not and never was a crime....
"The investigation by science builds up empirical evidence that proves, step by step, that man-made carbon dioxide is causing the Earth to warm up."
The investigation is an abject failure proven wrong by simple observation...do you suppose there might be a reason why your "detective" didn't provide you with the actual observations to support his case...they were all available...of course he didn't because his whole case would have failed as he tried to make his first point as the observations show clearly that energy is not being trapped in the atmosphere...the amount of energy exiting the atmosphere is increasing...precisely the opposite of what your hypothesis predicts...yet another predictive failure..