SSDD
Gold Member
- Nov 6, 2012
- 16,672
- 1,968
- 280
Calm down and stop sounding like a bitter old man. You cannot name any process that is spontaneous. Please tell me what revisions or misquotes or misinterpretations I have made. You have made many revisions of science yourself.No..I have simply lost patience with you and your stupid, constant lying revisions of practically everything I have said. It ranks as the number one tedious aspect of trying to talk to you. I have never said anything like you claim as evidenced by your lack of any quote by me saying such a thing....
It is all bullshit all the time from you...
.
Again with the lies...I have given you, over the months numerous spontaneous processes...you know full well I have and yet you lie about it...Is simply telling the truth so foreign to you that you simply can't bring yourself to do it?
Examples of spontaneous processes are ice melting to water....the decay of radioisotopes...iron rusting...a solute dissolving into solution..the expansion of gasses....the movement of energy from warm to cool..and on and on and on...it isn't my fault that you can't grasp the difference between a process that requires outside intervention and one that doesn't....
And as to what you have reinterpreted, practically every physical law that has come up..
You claimed that this equation described a two way energy exchange.
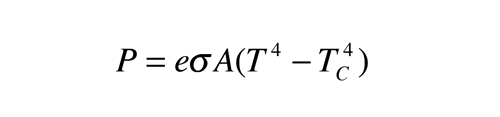
You claimed that this describes net energy flow
Second Law of Thermodynamics: It is not possible for heat to flow from a colder body to a warmer body without any workhaving been done to accomplish this flow. Energy will not flow spontaneously from a low temperature object to a higher temperature object
and again...on and on and on tedium ad nauseam...