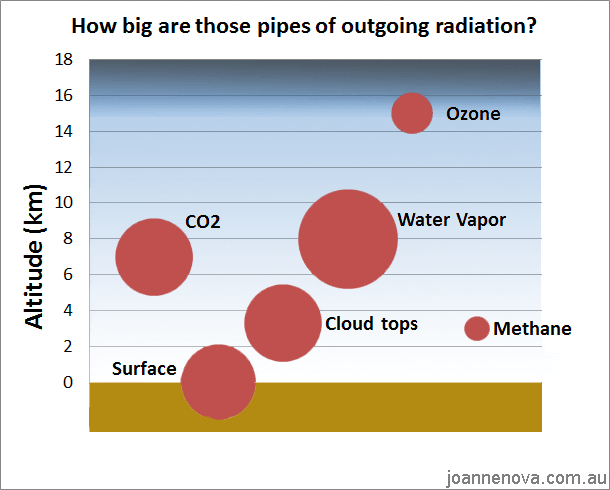
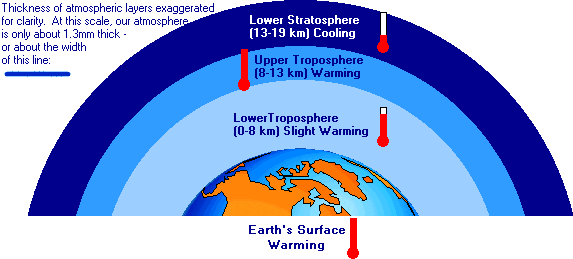
Could anyone inform me of what is going along with the measurement of CO2 (carbon dioxide) PPM (parts per million)?
It doesn't make sense to me. Seems like there are chemical units missing. Parts of CO2 per million of what?
I suppose the atmosphere isn't and can't be a continued constant of mixed molecules either, since single organisms and organic groups contribute progressively by their own developing standards.
I assume the information is widely available, since there have been so much attention to the problem, but the relay is apparently incomplete when reaching my position.
Parts-per notation - Wikipedia, the free encyclopedia
What does ppm or ppb mean?
Those links still don't answer the question.
If it is a comparison between concentrations, either current, past or future goal concentrations, those comparable concentrations should be made immediately evident.
Take the West Virginia University website as a reference for example.
Most fundamentally, it simply states a ppm is a conversion within the same unit system. One part of a million using the same measure.
The problem then with the CO2 in the atmosphere still remains, since although the same measure (liters for example) is being used to distinguish CO2 it is only CO2 which is being compared against itself.
If that approach would be complete and deliberate it would be partial and inaccurate science, and would then have to be categorized as short-sighted uneducated manipulation. The thought process of the alleged flawed scientists would have to be something like this: "in the place where I live there isn't as much air pollution (CO2) as many neighboring zones, so let us proceed in the attempt to make those areas with inappropriately high concentrations of CO2 as our cleaner spaces are, where the same pollution rate still hasn't affected us in our daily life in the same way as theirs, consequently not only assisting other communities in their development but also ensuring our own safe and comfortable future".
The frame of mind described above not only articulates scientific procedure absently, but also is a spawn of neglected arts and humanities.
The WVU has two examples that can be used in making the problem here presented clearer. Ink in water and salt on potatoes. The ppm in those examples is a comparison between two very distinct substances (ink and water, salt and potatoes), and what actually allows those to be compared is how they are capable of being measured; ink and water are liquid, so they can be measured through liters; salt and potatoes are solid, so they can be measured in grams.
Now, in this atmospheric CO2 problem here we only have half of the unit (measuring) balance needed for evaluation. Another substance of the same aspect as CO2 (gas) makes itself necessary, and especially a singular one, so that "ppm" (a single unit converter, according to WVU) can actually be used as primary comparing measurement or standard.
Joe Moma in a post above pointed to the possibility for two of those other substances being oxygen and nitrogen. However, oxygen and nitrogen aren't equivalent in aspect to CO2 (carbon dioxide). CO2 is a molecule, and oxygen and nitrogen are chemical elements.
The simplest compounds that can be made from oxygen and nitrogen is by putting two of each together (O2, N2), thereon making molecules.
Then the question would be to choose which of those (and possibly both) would be chosen for comparison.
200 ppm of CO2 by O2 and N2 standards?
For each million of combined O2 and N2, we have 200 of CO2?
With that information explicit the problem becomes simpler to analyze.
My questions next would be how stable (constant) are those concentrations by zone in which measurement has been approached, or how is it possible to calculate how stable and constant are the concentrations in the sum of all zones containing such concentrations, that is, a single global pattern for those concentrations?
I think I was informed at some point of some geological approaches for measuring the CO2 captured through centuries, but I cannot remember O2 and N2 also being addressed through the same method so that we could have a simple and mathematical analysis of the problem in reference to the past. Then would that geological method for measuring gas captured through centuries and millennia also be appropriate for measuring gas still circulating? If so, why?
Is the planet's atmosphere a closed molecular system in which a certain minimum and a certain maximum of specific molecule concentrations are necessarily bound to physical forces?
What are those maximums and minimums for each specific molecule according to regular modification of their chemical elements throughout standard physical conditions?
I suppose molecules can't escape gravity through classical physics, but what happens then to the molecules that have been geologically trapped? If we are dealing with a closed system, how are those trapped molecules to return to the atmosphere in it's due cyclical time? What about molecules that are not trapped, but are temporarily used by varying individual organisms? How do they affect the molecules and their concentrations?
These questions are more so relevant because they will provide evidence that the scientific method used in the prior investigations is actually to be agreed with.
I guess the situation here becomes less so about temperature modification than about toxicity analysis, and the problem is still equally relevant, although the politics of it aren't anymore confrontational since temperature is of a personal preference for differing individuals (even in an evolutionary scale), but it is toxicity which presents itself as biologically intrusive.