Wuwei
Gold Member
- Apr 18, 2015
- 5,342
- 1,178
- 255
Efficiency is a big part of the real world. The most efficient way to enclose area with a defined circumference is a circle. The radius is equal in all directions. A square is not as good but still better than a rectangle.
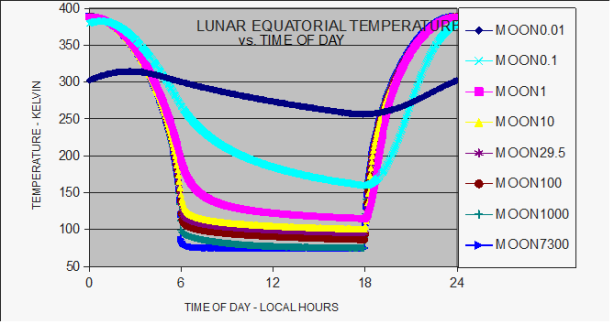
The moon receives as much solar energy as the Earth so why isn't it as warm? The main reason is the length of a lunar day/night cycle. It is very hot in the day and very cold at night. The energy inputs and outputs are extreme, less efficient. Like how a circle has more area per circumference than a flat rectangle. Only more so, because area is a ^2 relationship and temperature is a ^4 relationship. If the lunar 'day' was half as long the average temperature would be warmer. If you halved it again, warmer still. And so on, ignoring the effects of spinning the mass.
The predicted temperature of the Earth is -18C, from the average amount of sunlight hitting it. Now hold on a minute. We were talking about efficiencies. An Earth day is shorter than a lunar day but it is still 24 hours. Shouldn't the hypothetical max (circle) be greater than the actual Earth (square) which is greater than the Moon (rectangle)?
Obviously the atmosphere has something to do with it. But how does the atmosphere raise the average temperature ABOVE the theoretical maximum derived from the solar input?
I'll talk more about this if anyone is interested. Or we can just leave it as another unanswered question.
Hey Wuwei, you like theoretical problems.
Don't you find it interesting that the GHE is large enough to more than compensate for the inefficiencies caused by unequal input and output energies?
While it is just a variation on the theme of why the surface is warmer than the Sun's input, don't you find it amazing that entropy gained by converting sunlight to IR can 'power' such a large effect?
I realize the actual energy comes from radiation not lost to space but it seems to me that entropy gained in the total area causes entropy loss by the storage mechanism in a subset area.
I could be wrong. What do you think?
I looked at the idea of entropy for a rotating absorber (the moon). The following formula works for simple problems that are closed system, a solid (no atmosphere), and at equilibrium. The change in entropy is given by
delta S = n Cp log(Tf/Ti) where,
n = moles of moon surface that is warmed,
Cp = specific heat of surface,
Ti = initial temperature,
Tf = final temperature.
The factors in n x Cp are hard to determine. They refer to the mass and specific heat of the mass being heated.. They will be replaced by M referring to the amount of material being heated.
Dark side of moon Ti = 100 K
Bright side of moon Tf = 373 K
When looking at the sun hitting the moon there are two extremes where a quasi-equilibrium exists.
1. The moon is slowly rotating, and the sun is shining on one side for an extended period.
2. The moon is rapidly rotating, and night is so short that the temperature has no time to drop.
Case 1. The delta entropy for energy hitting the light side of the moon only.
delta S = M log(373K/100K) = M x 0.572
Case 2 . This is a bit trickier because there is no temperature data for the non-existing case of both sides getting half the energy all the time, but it can be computed from what we already know.
The sun energy E results in a temperature of Tf.
From SB equation, E = K Tf ^4. Where K contains all the proportionality constants.
Let T2 be the unknown Case 2 temperature of the moon.
The whole moon will be receiving half the energy. 2 x E = K T2^4.
Using simple algebra, Tf = T2 x fourth root of 2 or, T2 = Tf x 0.841
The entropy formula for case 2 is
delta S = 2 M log(Tf x 0.841 / Ti) = 2 M log( 3.137) = 2 M x .496
Note: the leading factor of 2 is because twice as much material (both sides) are being heated.
Summary for the moon:
Case 1 delta S = M .572
Case 2 delta S = M .992
The Case 1 slow spin is almost twice as efficient in using energy from the sun.
The earth, it is more like the fast spin Case 2 since both sides of the earth are closer in temperature. Case 2 is a less efficient mechanism for gaining energy from the sun, but at least we don't freeze or boil to death.
Your intuition seems to be right, entropy shows an inefficiency to the storage mechanism.