at the hospitals in the OP?Nope. Don't know the specific vaccinations that are required. We do know from the link that some vaccinations are required though. There is a phone number in the link to the office that handles volunteers. Give them a call tomorrow if you need to know specifically which ones. My only purpose was to show that requiring vaccinations to work in a hospital is nothing new.
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.

Note: This feature currently requires accessing the site using the built-in Safari browser.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Heroes Work Here
- Thread starter CarlinAnnArbor
- Start date
The only time in 40 years, republicans cared about people who work for a living.
The courts are not on their side.
August 6, 2021
Hospitals do have solid legal ground to stand on when it comes to requiring employees to get the COVID-19 vaccine, according to legal experts.
On Thursday, it was announced some of the largest healthcare systems in the Tri-State will require vaccinations for their employees.
But can hospitals require this?
The short answer, according to an attorney who specializes in employment law, is yes.
Legal experts point to a case in Houston last month where more than 150 health care workers who did not comply with a vaccine mandate were either fired or resigned.
The employees sued but a federal judge ruled in favor of the hospitals.
“That federal judge did not mince words at all,” stated FOX19 NOW Legal Expert Mike Allen. “He not only dismissed the case, he dismissed it with some pretty strong language.”
The courts are not on their side.
August 6, 2021
Hospitals do have solid legal ground to stand on when it comes to requiring employees to get the COVID-19 vaccine, according to legal experts.
On Thursday, it was announced some of the largest healthcare systems in the Tri-State will require vaccinations for their employees.
But can hospitals require this?
The short answer, according to an attorney who specializes in employment law, is yes.
Legal experts point to a case in Houston last month where more than 150 health care workers who did not comply with a vaccine mandate were either fired or resigned.
The employees sued but a federal judge ruled in favor of the hospitals.
“That federal judge did not mince words at all,” stated FOX19 NOW Legal Expert Mike Allen. “He not only dismissed the case, he dismissed it with some pretty strong language.”
you need a hep c vaccine etc....nurses who deny since need to go and it was not the left that mock them and denied covid...now was it?
Stryder50
Platinum Member
Almost all of those vaccines have years of testing and use, with essential "adjustments" to formulation, meaning their effectiveness is a larger "known" than that of the three Covid vaccines.Here is a list of requirements from before the virus for people to even volunteer at a local hospital.
View attachment 541985
Note also this from you excerpt: " or proof that you have had the disease," ... so far this qualification is not part of the pending and imposed mandates regards the covid vaccines. There lies my largest objection since both the wife and I have had covid. Injury to insult is how there are indications the vaccine will weaken our immune systems which are already stronger against covid than any of the vaccines would provide. So in our case, this "experimental" vaccine(s) could do us more harm than good.
Natural Immunity Longer Lasting Than Protection From COVID-19 Vaccines: Dr. Robert Malone

Natural Immunity Longer Lasting Than Protection From COVID-19 Vaccines: Dr. Robert Malone
The immunity conferred by recovering from COVID-19 is better than the protection afforded by COVID-19 vaccines, a prominent ...
 www.theepochtimes.com
www.theepochtimes.com
Stryder50
Platinum Member
As your article shows, the paperwork for approval was prioritized = accelerated, but the usual years of testing we see with other "mandated" vaccines was bypassed. That is why many of us see these covid vaccines as "experimental". That staus underscored by the makers of the vaccines being absolved of any liability for "side effects" or other adverse reactions.It is not experimental (in fact it has been tested with several other vaccines) and it has gone through the normal testing needed for approval.
In addition we have long had vaccine mandates: for schools, healthcare workers, government workers in certain professions as well as private employers.
As I pointed out in prior post, vaccines for other conditions have a longer track record of testing and corrections before having become "mandated".
Further more, the covid vaccines are a non-typical path to vaccine design, being mRNA which as this very long article will show, is a process and concept which while have a few decades history in R&D, has only recently reached status that some think it could be used. There are not years of application data to back up how safe or effective they really are.
The tangled history of mRNA vaccines
Hundreds of scientists had worked on mRNA vaccines for decades before the coronavirus pandemic brought a breakthrough.
...
The mRNA vaccine idea had a more favourable reception in oncology circles, albeit as a therapeutic agent, rather than to prevent disease. Beginning with the work of gene therapist David Curiel, several academic scientists and start-up companies explored whether mRNA could be used to combat cancer. If mRNA encoded proteins expressed by cancer cells, the thinking went, then injecting it into the body might train the immune system to attack those cells.
Curiel, now at the Washington University School of Medicine in St Louis, Missouri, had some success in mice10. But when he approached Ambion about commercialization opportunities, he says, the firm told him: “We don’t see any economic potential in this technology.”
Another cancer immunologist had more success, which led to the founding of the first mRNA therapeutics company, in 1997. Eli Gilboa proposed taking immune cells from the blood, and coaxing them to take up synthetic mRNA that encoded tumour proteins. The cells would then be injected back into the body where they could marshal the immune system to attack lurking tumours.
...
In light of those results, some mRNA experts now consider pseudouridine an essential component of the technology — and so, they say, Karikó’s and Weissman’s discovery was one of the key enabling contributions that merits recognition and prizes. “The real winner here is modified RNA,” says Jake Becraft, co-founder and chief executive of Strand Therapeutics, a Cambridge-based synthetic-biology company working on mRNA-based therapeutics.
Not everyone is so certain. “There are multiple factors that may affect the safety and efficacy of an mRNA vaccine, chemical modification of mRNA is only one of them,” says Bo Ying, chief executive of Suzhou Abogen Biosciences, a Chinese company with an mRNA vaccine for COVID-19 now in late-stage clinical testing. (Known as ARCoV, the product uses unmodified mRNA.)
...
That initially disappointed many investors and onlookers, because a vaccine platform seemed to be less transformative and lucrative. By the beginning of 2020, Moderna had advanced nine mRNA vaccine candidates for infectious diseases into people for testing. None was a slam-dunk success. Just one had progressed to a larger-phase trial.
But when COVID-19 struck, Moderna was quick off the mark, creating a prototype vaccine within days of the virus’s genome sequence becoming available online. The company then collaborated with the US National Institute of Allergy and Infectious Diseases (NIAID) to conduct mouse studies and launch human trials, all within less than ten weeks.
BioNTech, too, took an all-hands-on-deck approach. In March 2020, it partnered with New York-based drug company Pfizer, and clinical trials then moved at a record pace, going from first-in-human testing to emergency approval in less than eight months.
Both authorized vaccines use modified mRNA formulated in LNPs. Both also contain sequences that encode a form of the SARS-CoV-2 spike protein that adopts a shape more amenable to inducing protective immunity. Many experts say that the protein tweak, devised by NIAID vaccinologist Barney Graham and structural biologists Jason McLellan at the University of Texas at Austin and Andrew Ward at Scripps, is also a prize-worthy contribution, albeit one that is specific to coronavirus vaccines, not mRNA vaccination as a general platform.
...
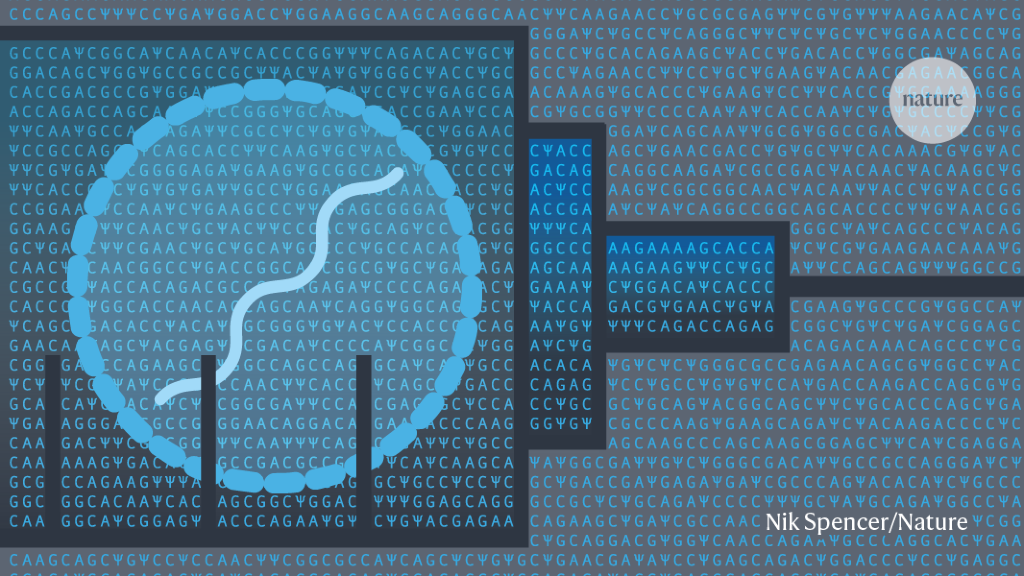
The tangled history of mRNA vaccines
Hundreds of scientists had worked on mRNA vaccines for decades before the coronavirus pandemic brought a breakthrough.
- Moderator
- #26
I see the point you are making but don’t agree with the conclusion that it is experimental simply because it doesn’t have as many rears of testing. It still has to meet a rigorous standard.As your article shows, the paperwork for approval was prioritized = accelerated, but the usual years of testing we see with other "mandated" vaccines was bypassed. That is why many of us see these covid vaccines as "experimental". That staus underscored by the makers of the vaccines being absolved of any liability for "side effects" or other adverse reactions.
As I pointed out in prior post, vaccines for other conditions have a longer track record of testing and corrections before having become "mandated".
Further more, the covid vaccines are a non-typical path to vaccine design, being mRNA which as this very long article will show, is a process and concept which while have a few decades history in R&D, has only recently reached status that some think it could be used. There are not years of application data to back up how safe or effective they really are.
The tangled history of mRNA vaccines
Hundreds of scientists had worked on mRNA vaccines for decades before the coronavirus pandemic brought a breakthrough.
...
The mRNA vaccine idea had a more favourable reception in oncology circles, albeit as a therapeutic agent, rather than to prevent disease. Beginning with the work of gene therapist David Curiel, several academic scientists and start-up companies explored whether mRNA could be used to combat cancer. If mRNA encoded proteins expressed by cancer cells, the thinking went, then injecting it into the body might train the immune system to attack those cells.
Curiel, now at the Washington University School of Medicine in St Louis, Missouri, had some success in mice10. But when he approached Ambion about commercialization opportunities, he says, the firm told him: “We don’t see any economic potential in this technology.”
Another cancer immunologist had more success, which led to the founding of the first mRNA therapeutics company, in 1997. Eli Gilboa proposed taking immune cells from the blood, and coaxing them to take up synthetic mRNA that encoded tumour proteins. The cells would then be injected back into the body where they could marshal the immune system to attack lurking tumours.
...
In light of those results, some mRNA experts now consider pseudouridine an essential component of the technology — and so, they say, Karikó’s and Weissman’s discovery was one of the key enabling contributions that merits recognition and prizes. “The real winner here is modified RNA,” says Jake Becraft, co-founder and chief executive of Strand Therapeutics, a Cambridge-based synthetic-biology company working on mRNA-based therapeutics.
Not everyone is so certain. “There are multiple factors that may affect the safety and efficacy of an mRNA vaccine, chemical modification of mRNA is only one of them,” says Bo Ying, chief executive of Suzhou Abogen Biosciences, a Chinese company with an mRNA vaccine for COVID-19 now in late-stage clinical testing. (Known as ARCoV, the product uses unmodified mRNA.)
...
That initially disappointed many investors and onlookers, because a vaccine platform seemed to be less transformative and lucrative. By the beginning of 2020, Moderna had advanced nine mRNA vaccine candidates for infectious diseases into people for testing. None was a slam-dunk success. Just one had progressed to a larger-phase trial.
But when COVID-19 struck, Moderna was quick off the mark, creating a prototype vaccine within days of the virus’s genome sequence becoming available online. The company then collaborated with the US National Institute of Allergy and Infectious Diseases (NIAID) to conduct mouse studies and launch human trials, all within less than ten weeks.
BioNTech, too, took an all-hands-on-deck approach. In March 2020, it partnered with New York-based drug company Pfizer, and clinical trials then moved at a record pace, going from first-in-human testing to emergency approval in less than eight months.
Both authorized vaccines use modified mRNA formulated in LNPs. Both also contain sequences that encode a form of the SARS-CoV-2 spike protein that adopts a shape more amenable to inducing protective immunity. Many experts say that the protein tweak, devised by NIAID vaccinologist Barney Graham and structural biologists Jason McLellan at the University of Texas at Austin and Andrew Ward at Scripps, is also a prize-worthy contribution, albeit one that is specific to coronavirus vaccines, not mRNA vaccination as a general platform.
...

The tangled history of mRNA vaccines
Hundreds of scientists had worked on mRNA vaccines for decades before the coronavirus pandemic brought a breakthrough.www.nature.com
Republicans claim the vaccine wasn't FDA approved, that's why they didn't take it.As your article shows, the paperwork for approval was prioritized = accelerated, but the usual years of testing we see with other "mandated" vaccines was bypassed. That is why many of us see these covid vaccines as "experimental". That staus underscored by the makers of the vaccines being absolved of any liability for "side effects" or other adverse reactions.
As I pointed out in prior post, vaccines for other conditions have a longer track record of testing and corrections before having become "mandated".
Further more, the covid vaccines are a non-typical path to vaccine design, being mRNA which as this very long article will show, is a process and concept which while have a few decades history in R&D, has only recently reached status that some think it could be used. There are not years of application data to back up how safe or effective they really are.
The tangled history of mRNA vaccines
Hundreds of scientists had worked on mRNA vaccines for decades before the coronavirus pandemic brought a breakthrough.
...
The mRNA vaccine idea had a more favourable reception in oncology circles, albeit as a therapeutic agent, rather than to prevent disease. Beginning with the work of gene therapist David Curiel, several academic scientists and start-up companies explored whether mRNA could be used to combat cancer. If mRNA encoded proteins expressed by cancer cells, the thinking went, then injecting it into the body might train the immune system to attack those cells.
Curiel, now at the Washington University School of Medicine in St Louis, Missouri, had some success in mice10. But when he approached Ambion about commercialization opportunities, he says, the firm told him: “We don’t see any economic potential in this technology.”
Another cancer immunologist had more success, which led to the founding of the first mRNA therapeutics company, in 1997. Eli Gilboa proposed taking immune cells from the blood, and coaxing them to take up synthetic mRNA that encoded tumour proteins. The cells would then be injected back into the body where they could marshal the immune system to attack lurking tumours.
...
In light of those results, some mRNA experts now consider pseudouridine an essential component of the technology — and so, they say, Karikó’s and Weissman’s discovery was one of the key enabling contributions that merits recognition and prizes. “The real winner here is modified RNA,” says Jake Becraft, co-founder and chief executive of Strand Therapeutics, a Cambridge-based synthetic-biology company working on mRNA-based therapeutics.
Not everyone is so certain. “There are multiple factors that may affect the safety and efficacy of an mRNA vaccine, chemical modification of mRNA is only one of them,” says Bo Ying, chief executive of Suzhou Abogen Biosciences, a Chinese company with an mRNA vaccine for COVID-19 now in late-stage clinical testing. (Known as ARCoV, the product uses unmodified mRNA.)
...
That initially disappointed many investors and onlookers, because a vaccine platform seemed to be less transformative and lucrative. By the beginning of 2020, Moderna had advanced nine mRNA vaccine candidates for infectious diseases into people for testing. None was a slam-dunk success. Just one had progressed to a larger-phase trial.
But when COVID-19 struck, Moderna was quick off the mark, creating a prototype vaccine within days of the virus’s genome sequence becoming available online. The company then collaborated with the US National Institute of Allergy and Infectious Diseases (NIAID) to conduct mouse studies and launch human trials, all within less than ten weeks.
BioNTech, too, took an all-hands-on-deck approach. In March 2020, it partnered with New York-based drug company Pfizer, and clinical trials then moved at a record pace, going from first-in-human testing to emergency approval in less than eight months.
Both authorized vaccines use modified mRNA formulated in LNPs. Both also contain sequences that encode a form of the SARS-CoV-2 spike protein that adopts a shape more amenable to inducing protective immunity. Many experts say that the protein tweak, devised by NIAID vaccinologist Barney Graham and structural biologists Jason McLellan at the University of Texas at Austin and Andrew Ward at Scripps, is also a prize-worthy contribution, albeit one that is specific to coronavirus vaccines, not mRNA vaccination as a general platform.
...

The tangled history of mRNA vaccines
Hundreds of scientists had worked on mRNA vaccines for decades before the coronavirus pandemic brought a breakthrough.www.nature.com
Now that it is FDA approved, now they claim there wasn't enough time in the approval process, so they resist, again.
Well, they didn't have an issue with the polio vaccine.
Salk's vaccine was then used in a test called the Francis Field Trial, led by Thomas Francis, the largest medical experiment in history at that time. The test began with about 4,000 children at Franklin Sherman Elementary School in McLean, Virginia,] and eventually involved 1.8 million children, in 44 states from Maine to California.
By the conclusion of the study, roughly 440,000 received one or more injections of the vaccine, about 210,000 children received a placebo, consisting of harmless culture media, and 1.2 million children received no vaccination and served as a control group, who would then be observed to see if any contracted polio.
The results of the field trial were announced 12 April 1955 (the tenth anniversary of the death of President Franklin D. Roosevelt, whose paralytic illness was generally believed to have been caused by polio). The Salk vaccine had been 60–70% effective against PV1 (poliovirus type 1), over 90% effective against PV2 and PV3, and 94% effective against the development of bulbar polio.
Soon after Salk's vaccine was licensed in 1955, children's vaccination campaigns were launched. In the U.S, following a mass immunization campaign promoted by the March of Dimes, the annual number of polio cases fell from 35,000 in 1953 to 5,600 by 1957. By 1961 only 161 cases were recorded in the United States.
Still scared of the polio vaccine?
Stryder50
Platinum Member
First off, at @71 years of age, I'm among those children back in the mid 1950s whom first got this vaccine and were the "guinea pigs" it was "experimented" with. I've gotten boosters ever since, so no way "Still scared of the polio vaccine?".Republicans claim the vaccine wasn't FDA approved, that's why they didn't take it.
Now that it is FDA approved, now they claim there wasn't enough time in the approval process, so they resist, again.
Well, they didn't have an issue with the polio vaccine.
Salk's vaccine was then used in a test called the Francis Field Trial, led by Thomas Francis, the largest medical experiment in history at that time. The test began with about 4,000 children at Franklin Sherman Elementary School in McLean, Virginia,] and eventually involved 1.8 million children, in 44 states from Maine to California.
By the conclusion of the study, roughly 440,000 received one or more injections of the vaccine, about 210,000 children received a placebo, consisting of harmless culture media, and 1.2 million children received no vaccination and served as a control group, who would then be observed to see if any contracted polio.
The results of the field trial were announced 12 April 1955 (the tenth anniversary of the death of President Franklin D. Roosevelt, whose paralytic illness was generally believed to have been caused by polio). The Salk vaccine had been 60–70% effective against PV1 (poliovirus type 1), over 90% effective against PV2 and PV3, and 94% effective against the development of bulbar polio.
Soon after Salk's vaccine was licensed in 1955, children's vaccination campaigns were launched. In the U.S, following a mass immunization campaign promoted by the March of Dimes, the annual number of polio cases fell from 35,000 in 1953 to 5,600 by 1957. By 1961 only 161 cases were recorded in the United States.
Still scared of the polio vaccine?
BTW, sort of typical strawman(person?) argument you have presented.
I notice most of your post appears to be a 'copy-paste' from elsewhere, but as could be expected from Leftist Loonie~pseudo-liberals, you fail on the intellectual integrity and plagiarize aspects. You did not clarify what parts of your post were "copy-paste" nor did you provide a citation of source, or link/URL.
That shows a significant shortfall on the intellectual integrity scale, and honest and ethics, and also opens the ownership of this Forum(USMB) to charges of copyright infringement. Hopefully the moderators will deal with you on this, but if they don't I'll bring it to their attention.
Would seem this is where you got your plagiarized content, based on text and embedded click links;
Polio vaccine - Wikipedia
You've also been a bit selective in the excerpt you presented and this section of the Wiki article is also worth noting, EXCERPT:
...
Safety incidents
In April 1955, soon after mass polio vaccination began in the US, the Surgeon General began to receive reports of patients who contracted paralytic polio about a week after being vaccinated with Salk polio vaccine from the Cutter pharmaceutical company, with the paralysis limited to the limb the vaccine was injected into. The Cutter vaccine had been used in vaccinating 200,000 children in the western and midwestern United States.[94] Later investigations showed that the Cutter vaccine had caused 40,000 cases of polio, killing 10.[94] In response the Surgeon General pulled all polio vaccines made by Cutter Laboratories from the market, but not before 250 cases of paralytic illness had occurred. Wyeth polio vaccine was also reported to have paralyzed and killed several children. It was soon discovered that some lots of Salk polio vaccine made by Cutter and Wyeth had not been properly inactivated, allowing live poliovirus into more than 100,000 doses of vaccine. In May 1955, the National Institutes of Health and Public Health Services established a Technical Committee on Poliomyelitis Vaccine to test and review all polio vaccine lots and advise the Public Health Service as to which lots should be released for public use. These incidents reduced public confidence in polio vaccine, leading to a drop in vaccination rates.[95]...

Polio vaccine - Wikipedia
As one whom has retired with a background in Quality Control(QC) & Quality Assurance(QA) this is what concerns me since I've yet to see convincing evidence of the QC/QA applications with these vaccines (three so-far) for Covid.
Also, as the Wiki article points out, polio vaccine was decades in development and had several years of use and "testing" before being "mandated" at local/state/national levels. Our three Covid vaccines have had only several months of rushed development and testing and bureaucratic certification before being "mandated"(forced) upon the public, and especially most* guv'mint and private sector employees.
*Most curious that USPS - Postal Workers, aren't mandated to have the vaccine to keep working, yet they are some of the government employed with the largest level of public contact and interaction. Care to guess how many USPS hands have touched that envelope you just got out of your mailbox and opened?
To be blunt "Smokin' OP", when it comes to honesty, integrity, ethics, and avoiding plagiarism you score not a ZERO, but a diving down into the negative numbers scale of the range. You have rapidly lost credibilty and done a great job of displaying either ignorance, intend to mis-lead and dis-inform, failure to protect the Forum from litigation potential, or all the above
So, you prove that by repeating the same sources as what I did?First off, at @71 years of age, I'm among those children back in the mid 1950s whom first got this vaccine and were the "guinea pigs" it was "experimented" with. I've gotten boosters ever since, so no way "Still scared of the polio vaccine?".
BTW, sort of typical strawman(person?) argument you have presented.
I notice most of your post appears to be a 'copy-paste' from elsewhere, but as could be expected from Leftist Loonie~pseudo-liberals, you fail on the intellectual integrity and plagiarize aspects. You did not clarify what parts of your post were "copy-paste" nor did you provide a citation of source, or link/URL.
That shows a significant shortfall on the intellectual integrity scale, and honest and ethics, and also opens the ownership of this Forum(USMB) to charges of copyright infringement. Hopefully the moderators will deal with you on this, but if they don't I'll bring it to their attention.
Would seem this is where you got your plagiarized content, based on text and embedded click links;

Polio vaccine - Wikipedia
en.wikipedia.org
You've also been a bit selective in the excerpt you presented and this section of the Wiki article is also worth noting, EXCERPT:
...
Safety incidents
In April 1955, soon after mass polio vaccination began in the US, the Surgeon General began to receive reports of patients who contracted paralytic polio about a week after being vaccinated with Salk polio vaccine from the Cutter pharmaceutical company, with the paralysis limited to the limb the vaccine was injected into. The Cutter vaccine had been used in vaccinating 200,000 children in the western and midwestern United States.[94] Later investigations showed that the Cutter vaccine had caused 40,000 cases of polio, killing 10.[94] In response the Surgeon General pulled all polio vaccines made by Cutter Laboratories from the market, but not before 250 cases of paralytic illness had occurred. Wyeth polio vaccine was also reported to have paralyzed and killed several children. It was soon discovered that some lots of Salk polio vaccine made by Cutter and Wyeth had not been properly inactivated, allowing live poliovirus into more than 100,000 doses of vaccine. In May 1955, the National Institutes of Health and Public Health Services established a Technical Committee on Poliomyelitis Vaccine to test and review all polio vaccine lots and advise the Public Health Service as to which lots should be released for public use. These incidents reduced public confidence in polio vaccine, leading to a drop in vaccination rates.[95]
...
~~~~~~~~~~~~~~~~
Polio vaccine - Wikipedia
en.wikipedia.org
As one whom has retired with a background in Quality Control(QC) & Quality Assurance(QA) this is what concerns me since I've yet to see convincing evidence of the QC/QA applications with these vaccines (three so-far) for Covid.
Also, as the Wiki article points out, polio vaccine was decades in development and had several years of use and "testing" before being "mandated" at local/state/national levels. Our three Covid vaccines have had only several months of rushed development and testing and bureaucratic certification before being "mandated"(forced) upon the public, and especially most* guv'mint and private sector employees.
*Most curious that USPS - Postal Workers, aren't mandated to have the vaccine to keep working, yet they are some of the government employed with the largest level of public contact and interaction. Care to guess how many USPS hands have touched that envelope you just got out of your mailbox and opened?
To be blunt "Smokin' OP", when it comes to honesty, integrity, ethics, and avoiding plagiarism you score not a ZERO, but a diving down into the negative numbers scale of the range. You have rapidly lost credibilty and done a great job of displaying either ignorance, intend to mis-lead and dis-inform, failure to protect the Forum from litigation potential, or all the above
Vaccinating children were the test trials.
But now you deflect when it comes to the covid vaccine.
Typical RW wing nut job.
Everyone here could be committing copyright infringement, including you.
IF the creator decides to push the issue.
If you download an image and post it, whether it be on your site, in a blog post, or on social media, you're likely committing copyright infringement. Copying any images or user-generated content without the creator's permission can constitute infringement, even if you link back to their website or original post.
"What you may be doing is committing copyright infringement and, because you gave attribution and a link, you admitted it and you told them about it," Clark said. She said that she has caught people copying her work in this fashion multiple times.
In addition to directly downloading and posting someone's work, copyright infringement can occur when you take an image from another company, like image-hosting companies like Getty. Some small business owners may assume that such a large company won't concern themselves with a minor offense, but this can be a reckless assumption. Getty has been known to send bills to sites that feature Getty's images but don't have a Getty subscription, for example.
The only person that can hold a business or individual accountable for copyright infringement is the original creator of the work. If, for example, you download or copy an image and the user doesn't notice, doesn't care or decides not to act, you don't have to worry. Relying on this is not a good practice, because either way you're exposed to serious risk.
You don't know WTF you're talking about, typical of republicans.
Just like vaccines.
Deplorable Yankee
Diamond Member
This is disgusting.....
Similar threads
- Replies
- 0
- Views
- 27
- Replies
- 21
- Views
- 189
- Replies
- 20
- Views
- 208
- Replies
- 93
- Views
- 395
Latest Discussions
- Replies
- 210
- Views
- 2K
- Replies
- 69
- Views
- 314
- Replies
- 37
- Views
- 154
Forum List
-
-
-
-
-
Political Satire 8037
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
ObamaCare 781
-
-
-
-
-
-
-
-
-
-
-
Member Usernotes 468
-
-
-
-
-
-
-
-
-
-
