Billy_Bob
Diamond Member
Quantum Mechanics is primarily theroy as observations are impossible. Given your description above there should then be NO HEAT generated but this is not the case as empirical evidence proves otherwise. So where does your heat come from if there is no loss? The molecules can not warm up if there is no resistance and loss.You think no energy is lost with the emission of a photon?
That is correct. When an electron absorbs a photon and gains 10 eV, no energy is lost when it then emits a photon of 10 eV. Because energy is conserved.
Actually, Yes! Or there is the loss of full photons (IE: Receives 10 and outputs 6) but there must always be a loss which generates heat (vibration). As this is quantum theroy we really dont know the answer to this question with certainty.You think the energy is absorbed by the molecule, a photon is emitted, and no energy is lost in the process?
You think when a CO2 molecule gains 10 eV from a single photon, it can emit a single 9 eV photon and lose 1 eV, just because?
So tell me toddster, do you really think no energy whatsoever is lost?
So tell me SSDD, how does this energy magically get lost in your scenario?
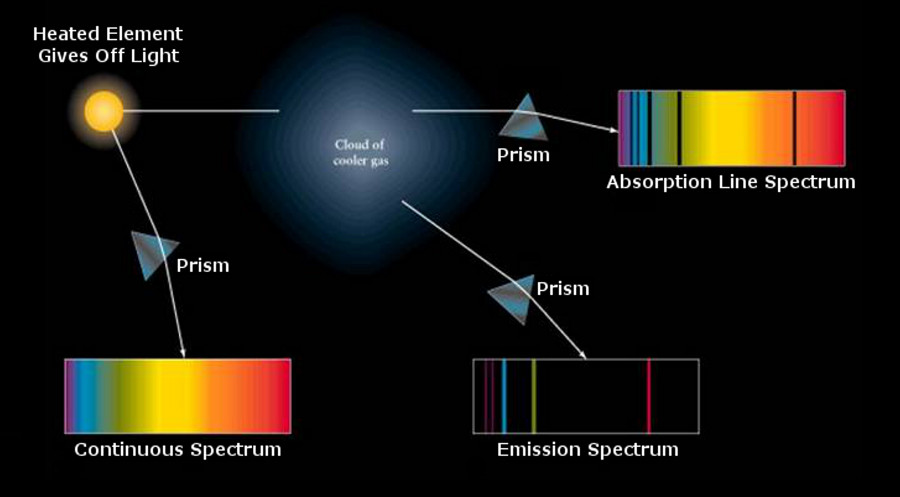
Absorption causes the molecule to vibrate (become excited) and the interaction with other adjacent molecules is what creates the heat. Absorption means conversion from the original emitted vibration pattern -> into the electron causing it to vibrate -> When enough energy is stored the electron then sheds a photon vibrating at the new molecular temperature. Again this is QM theroy being postulated.
The photon could very well be moving at 10 eV or it could be moving at 9 eV, we dont know for sure. What we do know for sure is that any time energy is absorbed or transferred there is loss. We also know that the mass emitting the new photon is what determines its vibration or frequency.
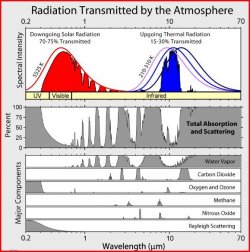
We know for instance that cylinder full of CO2 molecules, at 1000ppm using argon as its inert counter, bombarded by photons at 0.4um will emit at 6.0um to 13.0um which is the bandwidth that is not able to reabsorb the lower vibrating photons in CO2. The narrow spectrum generator was tested in several bandwidths always ending with the same result up to 2.2um. Curiously as we hit areas of non-cunduction/absorbtion (or total band pass) we registered scatter and reflection. We didn't register how much heat was built up in the gas but that is for another day.
Again I dont really care about the terms, just be skeptical and ask questions. Remember, theory is just that, Theory!
There are a lot of questions and very few answers. I'll butt out now.
Last edited: