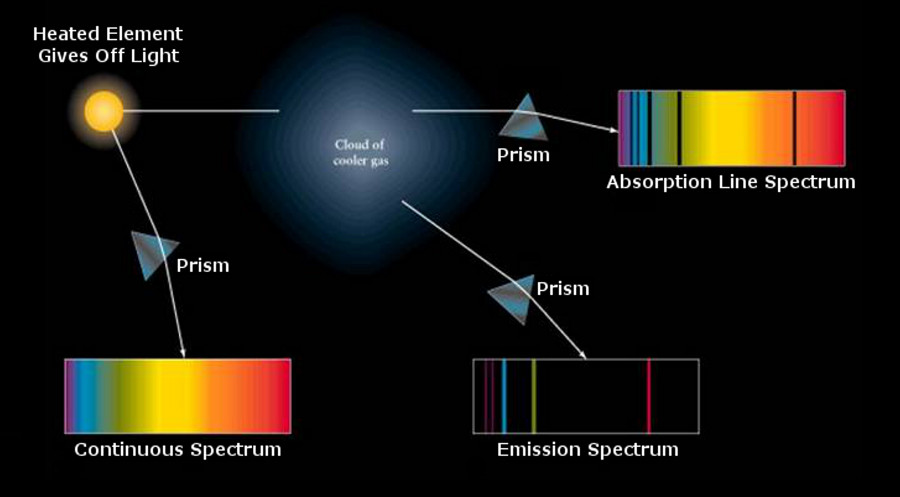
absorption equals emission.
If we were a single gas planet this would have meaning. We are not. Now lets add those other gases and see what happens.. This is precisely where AGW goes wrong. People get all giddy that CO2 passes and emits in specific wavelengths, and it may well be able to absorb that same wavelength (again QM theroy) again but they always leave out the other gases and water vapor. How they affect CO2 is not what they expected.
Water vapor is expected to increase temperature but what we have found in empirical evidence is that it has no bearing whatsoever in accelerating warming but it does accelerate cooling.
People get all giddy that CO2 passes and emits in specific wavelengths, and it may well be able to absorb that same wavelength
Wait, your original claim was wrong?




