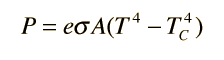Moron...the 2nd result is 6 meters...I merely forgot to change the figure for area in the equation generator. P is calculated for 6 square meters. What do you think is rediculous about that result?
That is the equation for the 1 m^2 speck emitting radiation
That is your equation for the 6 m^2 box radiating
First you have a typo and are missing a leading zero. Second, you forgot to multiply your second equation by the area of 6 m^2. If you did your answer would be
6 x 0.00571 W/m^2 = .03082 W/m^2
That is what is ridiculous. You are misusing your favorite equation.
.