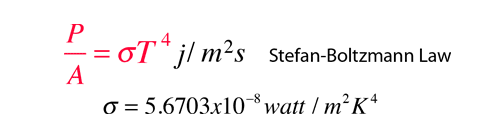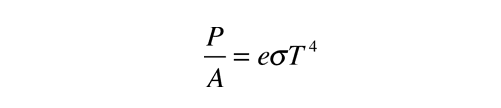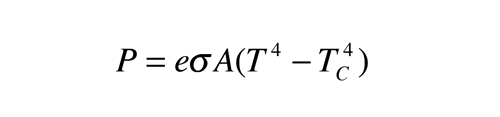CO2 that absorbs IR isn't warmed? Link?
Of course not...the equilibrium temperature of CO2 is about -80F, as a result, it emits everything it absorbs trying to reach its equilibrium temperature...if it doesn't lose the energy via collision that is.
Yes. IR which moves in all directions, even back toward the surface.
No..it only moves towards cooler areas...refer to the second law of thermodynamics which states that energy will not move SPONTANEOUSLY from a cool area to a warmer area
refer to the second law of thermodynamics which states that energy will not move SPONTANEOUSLY from a cool area to a warmer area
How does a photon know if it was created by work which would allow it to travel toward warmer matter?