....and keep using the entire amount under the 300 K curve instead of the portion which the CO2 actually absorbs:

It's not me who uses the total area under the curve. I specifically reference the different bands, some escape freely, some are retarded by water vapour, some by CO2. I wish you would directly quote any of my statements that you disagree with, rather than make a strawman caricature that you then attribute to me.
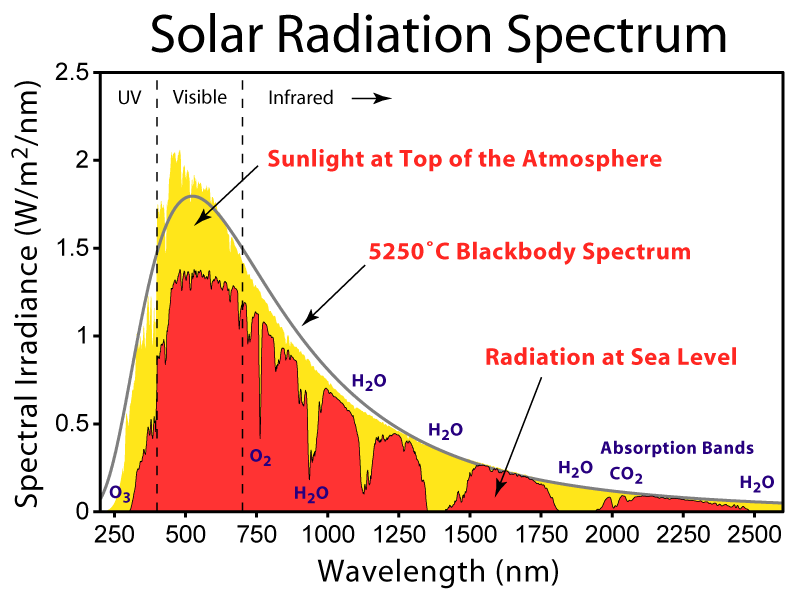
I notice you have not responded to the point I made about your graph. The energy output from the sun is four orders of magnitude greater in the visible light range than it is for the CO2 reactive IR band.
The IR coming off the Sun is a large amount but not in comparison to the total amount.
By the time the Sun's radiation reaches the Earth it has been attenuated to just 1360w at the zenith. The proportions of the wavelengths remains the same but the flux has been reduced. So the maximum CO2 reactive radiation reaching the Earth is 1360 divided by 10^4. That is further reduced by a factor of 4 to compensate for the rotation of the Earth. In actuality it should be further reduced for the inefficiencies involved with uneven distribution because of the rotation but that is splitting hairs. The amount of 15 micron radiation reaching the Earth is real but negligible. And it has already been accounted for in the amount of energy absorbed by the atmosphere.
It is a clever idea to think about but in reality it doesn't make much of a difference.