New gene tests for lung cancer may improve treatment...

Gene Tests May Improve Lung Cancer Care
May 30, 2014 ~ Nearly a million people die every year from lung cancer, and the World Health Organization expects the death rate to rise as more people take up cigarette smoking. Researchers may have made an important step toward controlling this disease and extending lives.

Gene Tests May Improve Lung Cancer Care
May 30, 2014 ~ Nearly a million people die every year from lung cancer, and the World Health Organization expects the death rate to rise as more people take up cigarette smoking. Researchers may have made an important step toward controlling this disease and extending lives.
Cigarette smoking is responsible for most lung cancers. Studies have established that connection. Others have established that inhaling toxins from someone else's cigarettes can also cause the disease. Nearly 1 million people die from lung cancer every year. Another 600,000 die from second-hand smoke. And, until recently, the treatment all patients received was the same chemotherapy. Sometimes it helped, but sometimes it didn't.
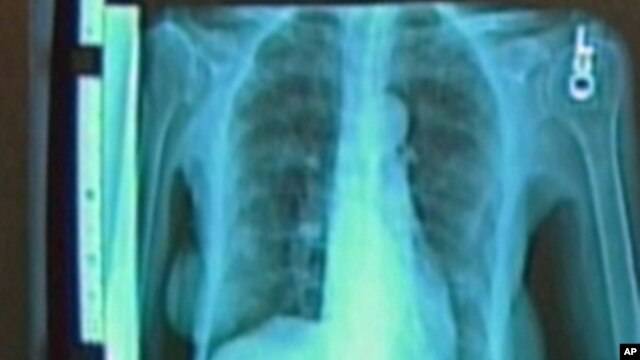
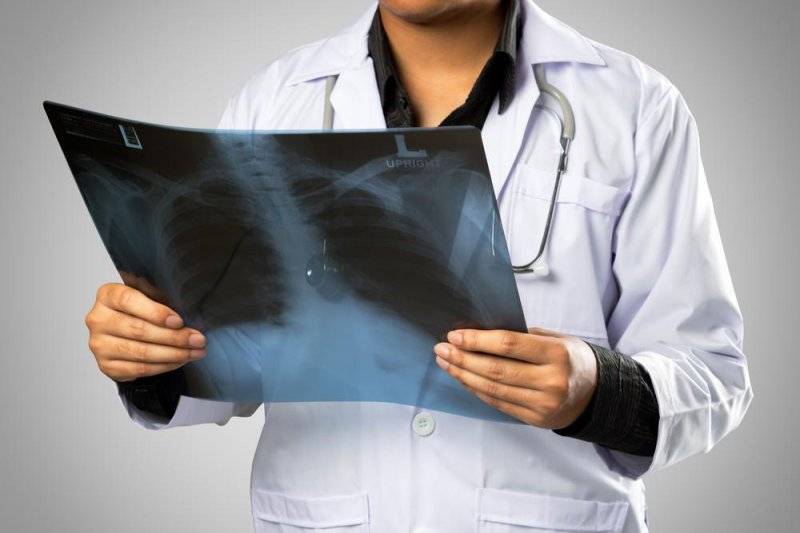
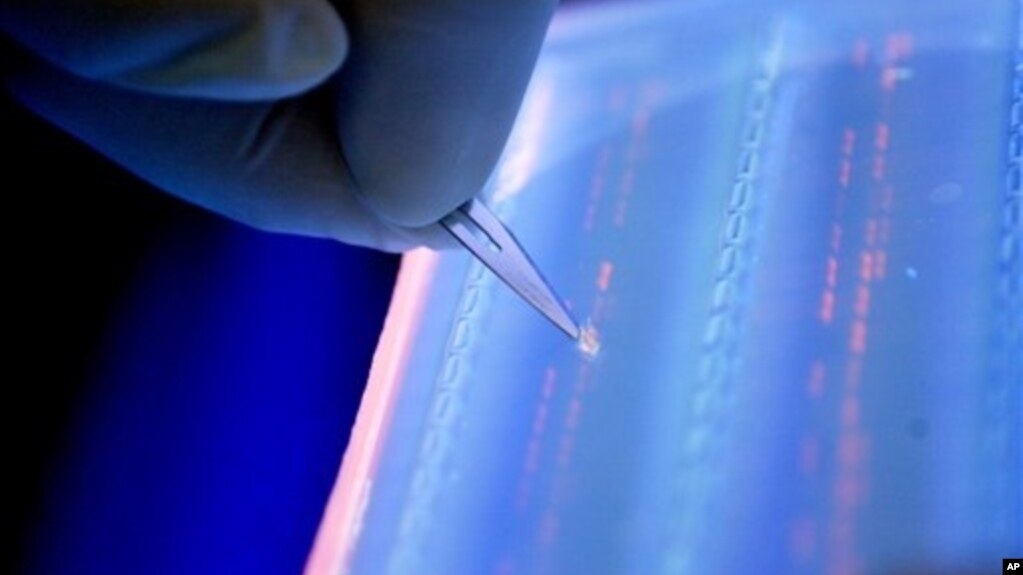
Researchers have been trying to come up with better treatments to shrink the tumors without affecting normal tissue or subjecting patients to the negative side-effects of chemotherapy. One new treatment relies on a test to identify damaged genes called oncogenes that allow cancer cells to grow and spread. Oncologist Mark Kris from Memorial Sloan Kettering Cancer Center led a study that examined the role oncogenes play in the spread of cancer. "That damaged gene makes certain proteins, and those proteins become absolutely critical to the growth of that cancer cell, said Kris.
Kris led a study involving 1,000 patients with advanced lung cancer, analyzing tumors for 10 different mutations that drive the spread of the disease. We had this vision that we could test the tumors of patients at the time they were diagnosed. We could test for 10 of these oncogenes that we know underlie lung cancer," said Kris. "We were able to find one of those driver oncogenes in 64 percent of the patients, and in 28 percent of the patients we were able to choose a therapy based on that information.
Identifying those damaged genes would allow doctors to better target treatment. Dr. Kris said the testing is fast, accurate and cost effective. The results showed that patients whose driver oncogenes were specifically targeted lived, on average, a year longer than those who received standard chemotherapy. In addition, drugs directed at the damaged genes cause fewer side-effects. Kris said the results of the study will help treat people with all types of cancer as doctors learn more about genes that drive the disease. The study was published in the Journal of the American Medical Association.
Gene Tests May Improve Lung Cancer Care