Trakar
VIP Member
- Feb 28, 2011
- 1,699
- 73
- 83
a chemist’s view
“A short history of ocean acidification science in the 20th century: a chemist’s view”
“A short history of ocean acidification science in the 20th century: a chemist’s view”
I have always enjoyed these types of reviews of a field of knowledge and understanding from the perspective of other specific concentrations of knowledge and understanding. With this particular field of science, I most often focus on the physics and maths of how solar energy interacts with the atmosphere and surface of our planet. It is interesting to follow the path from a different focus of science. Good read, recommended for all who are curious about this area of science and its growth in many diverse areas over the last century.Abstract
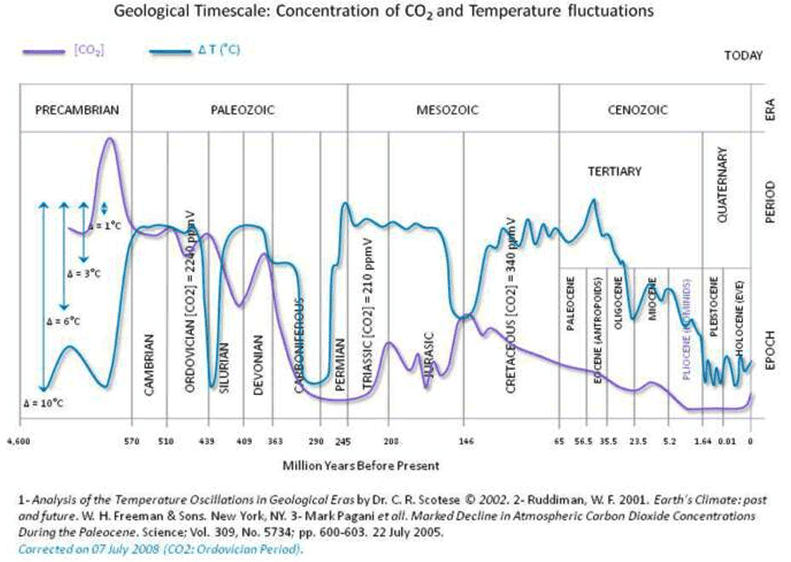
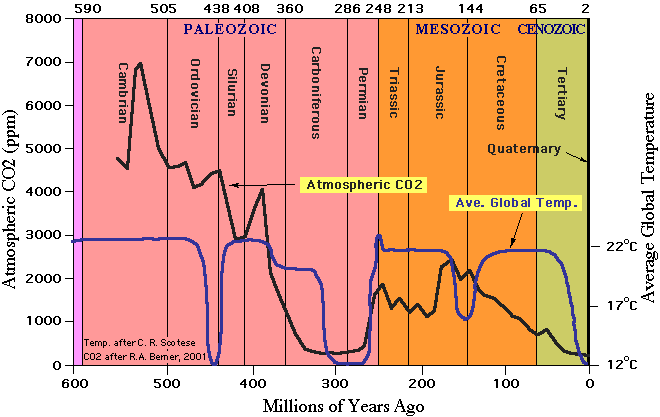
This review covers the development of ocean acidification science, with an emphasis on the creation of ocean chemical knowledge, through the course of the 20th century. This begins with the creation of the pH scale by Sørensen in 1909 and ends with the 5 widespread knowledge of the impact of the “High CO2 Ocean” by then well underway as the trajectory along the IPCC scenario pathways continues. By mid-century the massive role of the ocean in absorbing fossil fuel CO2 was known to specialists, but not appreciated by the greater scientific community. By the end of the century the trade-offs between the beneficial role of the ocean in absorbing some 90% of all heat 10 created, and the accumulation of some 50% of all fossil fuel CO2 emitted, and the impacts on marine life were becoming clear. This paper documents the evolution of knowledge throughout this period.
(…
Conclusion
…By the end of the 20th century until the seminal first “High CO2 Ocean” meeting in 2004 (Cicerone et al., 2004) a far busier scene had evolved. As the major IPCC Special Report on Carbon Capture and Storage was proceeding greatly increased awareness of the impacts of what is now known as “Ocean Acidification” was increasing. Objectively the fears over direct ocean CO2 disposal are unwarranted; it would be too expensive, too controversial, and technically challenging to transfer large quantities of CO2 to great depth. But, in any case such efforts would be dwarfed by the approximately 1 million tons of fossil fuel CO2 per hour now being transferred from air to sea; it is very doubtful that even 1% of this quantity (10 000 tons per hour) could be achieved by ships and pipelines. The “greater than 99% problem” – and the associated climate benefit – is the net air to sea flux. Nonetheless urgent discussions surrounding the concept of direct ocean CO2 disposal greatly aided the modern scientific understanding of the impacts of elevated CO2 on the ocean (Shirayama et al., 2004)…
Last edited: