Ghost1776
Diamond Member
- Dec 1, 2021
- 2,075
- 1,756
- 1,893
- Banned
- #1

Study Finds More Than 25% Increase in Emergency Cardiovascular Events in 16–39 Age Group During COVID-19 Vaccination Rollout in Israel | The Gateway Pundit | by Jim Hᴏft
A recent study in Israel revealed more than a 25% increase in calls in Israel’s National Emergency Medical Services (IEMS) concerning emergency cardiovascular events in the 16 to 39 age group during the Covid-19 vaccination rollout.
by Figs. 1 and 2 that present the graphs described in the “Methods” section for both CA and ACS, CA only, and ACS only, respectively. Both the CA and ACS call counts (red curve) start increasing early January 2021 and seem to track closely the 2nd dose curve (solid blue curve). They peak around early March and then decrease during March and the first part of April (Figs. 1B and 2B). The graphs also highlight the lack of association between the COVID-19 infection counts (grey curve) and the CA and ACS call counts, which is most clearly seen during the first two major infection waves in 2020.
Figure 1
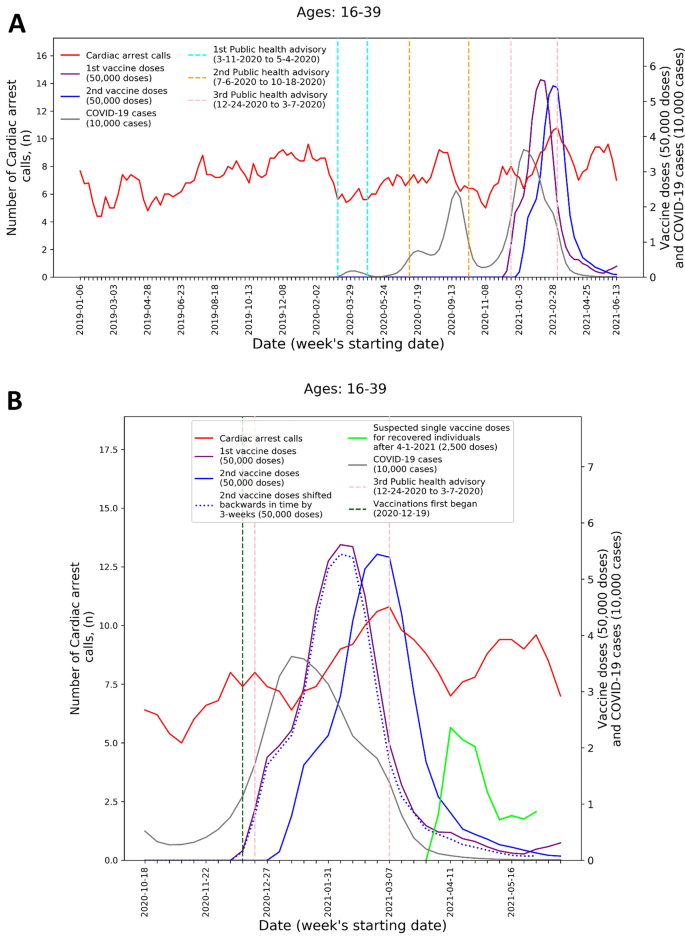
Weekly counts of cardiac arrest calls (five-week centered moving-average), COVID-19 cases (three-week centered moving-average), and vaccination doses (three-week centered moving-average) for those between 16 and 39 during: A) the study period (January 1st, 2019, to June 20th, 2021) and B) the third COVID-19 wave and vaccination distribution period (October 18th, 2020, to June 20th, 2021). COVID-19 Coronavirus disease 2019.
But feel safe and secure because a gov. Who changed information from the population and a Gov. Who hid information from the public expects you to believe this truth now LOL……… Keep taking your jabs ppl they are safe and effective cause you know the FDA said so so it must be so.
Key Points:
- After conducting an updated analysis, evaluation and investigation of reported cases, the FDA has determined that the risk of thrombosis with thrombocytopenia syndrome (TTS), a syndrome of rare and potentially life-threatening blood clots in combination with low levels of blood platelets with onset of symptoms approximately one to two weeks following administration of the Janssen COVID-19 Vaccine, warrants limiting the authorized use of the vaccine.
- The FDA has determined that the known and potential benefits of the vaccine for the prevention of COVID-19 outweigh the known and potential risks for individuals 18 years of age and older for whom other authorized or approved COVID-19 vaccines are not accessible or clinically appropriate, and for individuals 18 years of age and older who elect to receive the Janssen COVID-19 Vaccine because they would otherwise not receive a COVID-19 vaccine.
- The Fact Sheet for Healthcare Providers Administering Vaccine now reflects the revision of the authorized use of the Janssen COVID-19 Vaccine and includes a warning statement at the beginning of the fact sheet for prominence which summarizes information on the risk for TTS. Additionally, information on the revision to the authorized use of the vaccine and updated information on this risk of blood clots with low levels of blood platelets has been added to the Fact Sheet for Recipients and Caregivers.

Coronavirus (COVID-19) Update: FDA Limits Use of Janssen COVID-19 Vaccine to Certain Individuals
The FDA has limited the authorized use of the Janssen COVID-19 Vaccine to individuals 18 older for whom other available COVID-19 vaccines are not accessible or clinically appropriate, and to individuals 18 and older who elect to receive it because they would otherwise not receive a COVID-19 vaccine.
Last edited: