SSDD
Gold Member
- Nov 6, 2012
- 16,672
- 1,968
- 280
- Thread starter
- #1,001
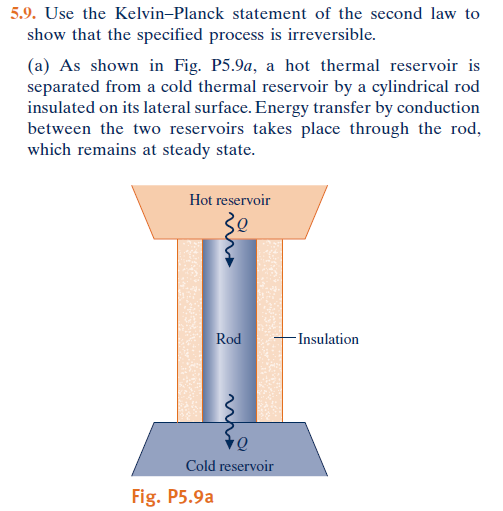
Nope you continue to torture the laws of physics that are in every textbook.Never happened...but the way you interpret and torture information in your mind, I have no doubt that you believe you have disproven.....something.
.
So you are saying that you agree with this statement precisely as it is written?:
Second Law of Thermodynamics: It is not possible for heat to flow from a colder body to a warmer body without any work having been done to accomplish this flow. Energy will not flow spontaneously from a low temperature object to a higher temperature object.
I agree with the original statement
Clausius Statement of the Second Law
One of the earliest statements of the Second Law of Thermodynamics was made by R. Clausius in 1850. He stated the following.
“It is impossible to construct a device which operates on a cycle and whose sole effect is the transfer of heat from a cooler body to a hotter body”.
Clausius Statement of the Second Law of Thermodynamics
There are many statements of the second law that are exactly that. Nothing more. Nothing less. Do you believe those?
Shuck and jive....dodge and weave...duck and cover.....what's the matter? Afraid to simply state that you are in disagreement with the second law of thermodynamics?
And you cherry picked your statement from a block of text...typical...How about we look at the whole statement...
One of the earliest statements of the Second Law of Thermodynamics was made by R. Clausius in 1850. He stated the following.
“It is impossible to construct a device which operates on a cycle and whose sole effect is the transfer of heat from a cooler body to a hotter body”.
Heat cannot spontaneously flow from cold system to hot system without external work being performed on the system. This is exactly what refrigerators and heat pumps accomplish. In a refrigerator, heat flows from cold to hot, but only when forced by an external work, refrigerators are driven by electric motors requiring work from their surroundings to operate.
So you agree with the Clausius statement...heat cannot spontaneously flow from a cold system to a hot system without external work being performed on the system? You agree with that statement? After all, you provided it.