The phys.org article mentions imaging
yada, yada
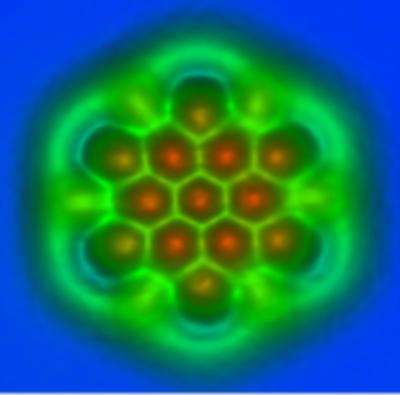
Buckyball -- "football shape" from above, i.e. shaped exactly like a soccer ball.
PAHS -- "two planar polycyclic aromatic hydrocarbons" resemble layered graphene
The context makes plain that the latter (two PAHS) are really what's shown in the image (and the video animation). The PAHS are not strictly planar, like layers of graphene, but apparently close enough for government work to study them as if the were. They float on the surface of a liquid (likely distilled water, but whatever) until one slides on top of the other. Then they snap a picture, purportedly to study bond strengths.
Anyways. Point being, we're seeing no atoms, nuclei, or bonds. We see modulation and backlighting tricks that make the hexagonal shapes stand out in stark contrast to the rest..
of the aromatic molecules. No "orange nucleus to each atom" -- Probably orangish light filtering through the empty center of each aromatic ring.