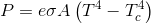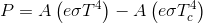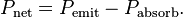Reference the inverse square law...
If you want to know why you lose heat faster in a freezer than you do in the cold refer to the Stefan Boltzman Law... which describes the amount of energy a radiator loses depending upon the temperature difference between the radiator and its cooler surroundings.
You never explained why a one-way flow of energy would go faster or slower, depending on the temperature of the surroundings. Do the photons (or waves, for those who don't believe) go faster, or slower, or is the difference in speed due to the number of photons (or waves) that are emitted? And how does the warmer object know how fast to emit?
Please explain how your theory makes sense, in relation to the Stefan Boltzman Law.
You really can't look at that equation and tell that it describes a one way flow of energy? If you can't, say so and I will explain but for all your pretention of knowing physics, one would think that you could look at that equation and tell it describes a one way energy flow from a warm radiator to cooler surroundings.
You really can't look at that equation and tell that it describes a one way flow of energy?
You really can't look at that equation and tell that it describes a net flow of energy?
one would think that you could look at that equation and tell it describes a one way energy flow from a warm radiator to cooler surroundings.
If energy only flows one way, why does the temperature of the cooler have any impact on the speed of that flow? My understanding of physics can explain that easily.
The Stefan-Boltzmann constant, symbolized by the lowercase Greek letter sigma ( ), is a physical constant involving black body radiation. A black body, also called an ideal radiator, is an object that radiates or absorbs energy with perfect efficiency at all electromagnetic wavelengths. The constant defines the power per unit area emitted by a black body as a function of its thermodynamic temperature .
And my understanding of physics fits with the Stefan-Boltzmann constant, your understanding needs an amendment, to explain why a black body sometimes stops emitting, if another, warmer object approaches.
Science 24 May 1963:
Vol. 140 no. 3569 pp. 870-877
DOI: 10.1126/science.140.3569.870
In a practical situation and room-temperature setting, humans lose considerable energy due to thermal radiation. However, the energy lost by emitting infrared light is partially regained by absorbing the heat flow due to conduction from surrounding objects, and the remainder resulting from generated heat through metabolism. Human skin has an emissivity of very close to 1.0 . Using the formulas below shows a human, having roughly 2 square meter in surface area, and a temperature of about 307 K, continuously radiates approximately 1000 watts. However, if people are indoors, surrounded by surfaces at 296 K, they receive back about 900 watts from the wall, ceiling, and other surroundings, so the net loss is only about 100 watts.
This Science article agrees with my understanding of physics, net flow, not one way.
If your understanding was correct, one way flow only, how could they have made such a huge error?
This was 1963, you can't blame the warmers.