Informally, it is the amount of
heat that must be added to one unit of mass of the substance in order to cause an increase of one unit in
temperature. The
SI unit of specific heat capacity is
joule per
kelvin per
kilogram, J⋅kg−1⋅K−1.
[1] For example, the heat required to raise the temperature of 1 kg of water by 1 K is 4184 joules, so the specific heat capacity of water is 4184 J⋅kg−1⋅K−1.
[2]
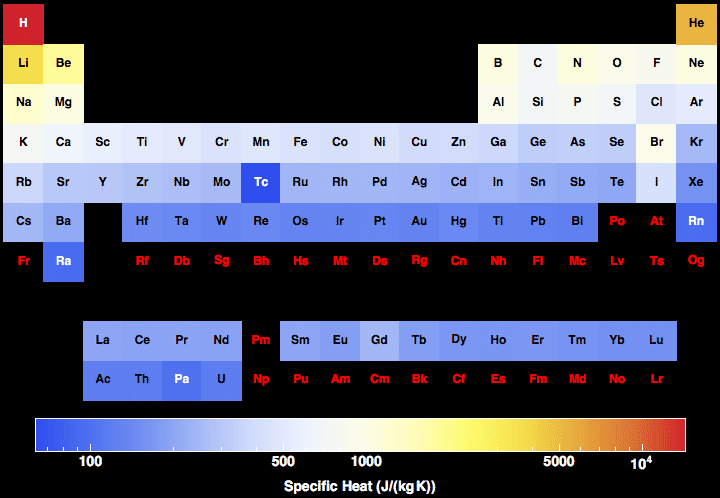
Specific heat capacity often varies with temperature, and is different for each
state of matter. Liquid water has one of the highest specific heat capacities among common substances, about 4184 J⋅kg−1⋅K−1 at 20 °C; but that of ice, just below 0 °C, is only 2093 J⋅kg−1⋅K−1. The specific heat capacities of
iron,
granite, and
hydrogen gas are about 449 J⋅kg−1⋅K−1, 790 J⋅kg−1⋅K−1, and 14300 J⋅kg−1⋅K−1, respectively.
[3] While the substance is undergoing a
phase transition, such as melting or boiling, its specific heat capacity is technically undefined, because the heat goes into changing its state rather than raising its temperature.
The specific heat capacity of a substance, especially a gas, may be significantly higher when it is allowed to expand as it is heated (specific heat capacity
at constant pressure) than when it is heated in a closed vessel that prevents expansion (specific heat capacity
at constant volume). These two values are usually denoted by {\displaystyle c_{p}}
and {\displaystyle c_{V}}
, respectively; their quotient {\displaystyle \gamma =c_{p}/c_{V}}
is the
heat capacity ratio.
The term
specific heat may also refer to the ratio between the specific heat capacities of a substance at a given temperature and of a reference substance at a reference temperature, such as water at 15 °C;
[4] much in the fashion of
specific gravity. Specific heat capacity is also related to other intensive measures of heat capacity with other denominators. If the amount of substance is measured as a number of
moles, one gets the
molar heat capacity instead, whose SI unit is joule per kelvin per mole, J⋅mol−1⋅K−1. If the amount is taken to be the
volume of the sample (as is sometimes done in engineering), one gets the
volumetric heat capacity, whose SI unit is joule per kelvin per
cubic meter, J⋅m−3⋅K−1.
One of the first scientists to use the concept was
Joseph Black, an 18th-century medical doctor and professor of medicine at
Glasgow University. He measured the specific heat capacities of many substances, using the term
capacity for heat.
[5]