Rigby5
Diamond Member
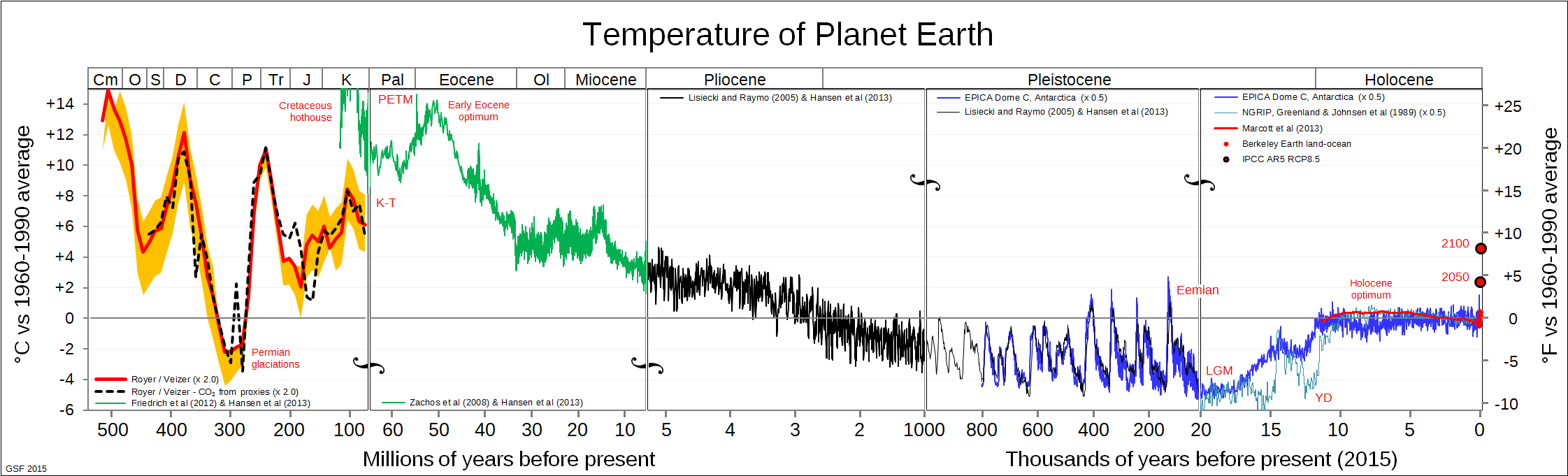
You have a point in that modern global warming is referring to the increase from the stasis point the planet has had for hundreds of millions of years.
And the warming of 40 degrees F from the theoretical temperature if there was no greenhouse gases, should not be confused with current warming, even though that was past global warming to get to the temperature the atmosphere was for hundreds of millions of years.
But even that is a generalization, but cause we also know the historic value is really the average of a 120,000 year long fluctuating cycle. But that is not really important, since we now are talking about a very fast, new, and different change being cause by man's release of hundreds of millions of years worth of sequestered carbon and solar energy, through the burning of fossil fuel. We don't even have to talk about the change in upper atmosphere radiation capability, when you simply consider all that sequestered, fossil, ancient solar energy being release so quickly.
You have a point in that modern global warming is referring to the increase from the stasis point the planet has had for hundreds of millions of years.
Stasis point?
Was that the temperature before the Little Ice Age?
Was that the temperature during the Little Ice Age?
Was that the temperature after the Little Ice Age?
Maybe provide a year for the "stasis point"?
And a definition for stasis point? Maybe a link?
We have had at least 12 ice ages that we know of, so we know the range of temperature swings.
That range, or the average of it, is a consistent state of stasis.
But now we have changed that.
According to the ice age cycles that are about 120,000 years long, right now the planet is supposed to be over its warming, and slightly into its cooling phase.
So now, "global warming" refers to the deviation from the normal 120,000 year long ice age cycle, above what it would be in the normal cycles.
It does not refer to the warming within the 120,000 year long cycle range.
And by the way, the most accepted theory for the 120,000 year long ice age cycle, is plants using up most of the CO2, that causing cooling, that kills the plants, they decompose, releasing the CO2 again, which then causes warming, that again induces high plant growth.
If you think plants can't do that and have that much effect, all you have to do is remember that even further back, the Earth has a toxic ammonia and methane atmosphere, and they theorize that it was micro organisms that converted the atmosphere to the current oxygen rich one we have now.
{...
After the hydrogen and helium had escaped, Earth's Hadean atmosphere was left with methane, ammonia, water vapor, and small percentages of nitrogen and carbon dioxide. A cataclysmic meteorite bombardment around 3.9 Ga kept much of the Earth's surface in the molten state, and the incoming impactors may have brought additional water, methane, ammonia, hydrogen sulfide and other gases that supplemented the atmosphere.
...
Microfossils of sulfur-metabolizing cells have been found in 3.4-billion-year-old rocks[6], and it is known that the first aquatic photosynthetic organisms originated around 3.5 Ga. The oxygen produced by cyanobacteria (blue-green algae) during the Archean Eon reacted with the metal ions in the anoxic sea. Billions of years would pass before the photosynthetic microorganisms could eventually change the composition of the atmosphere. By the middle of the Archean Eon, the Earth had cooled enough so that most of the water vapor in the atmosphere had condensed as water, and the Earth had its first days without clouds. Ammonia and methane were only minor constituents of the atmosphere. Carbon dioxide comprised about 15% of the atmosphere and the percentage of nitrogen was 75%.[5] In essence, most of the original components of the atmosphere had escaped, precipitated as liquids or reacted chemically to form solid compounds. The volcanic activity and the photosynthetic bacteria were now the major factors influencing the Earth's atmospheric composition.
Earth's third atmosphere (Proterozoic Eon, 2.5 to 0.54 Ga)
Monocellular life proliferated during the Proterozoic Eon. Anaerobic microbial life thrived in a planet with little oxygen. Anaerobic organisms obtained their energy in various ways. Methanogens combined hydrogen and carbon dioxide to produce methane and water:
CO2 + 4 H2CH4 + 2 H2O
Sulfate reducing bacteria combined methane and sulfate radicals:
CH4 + SO4--HCO3- + HS- + H2O
Other organisms capable of photosynthesis used the energy of sunlight to convert the abundant carbon dioxide and water into carbohydrates (C6H12O6) and oxygen, which was deadly to the anaerobes.
6 CO2 + 6 H2OC6H12O6 + 6 O2
Production of oxygen through photosynthesis
...}
Evolution of the Earth's Atmosphere
That range, or the average of it, is a consistent state of stasis.
Geez.
stasis: a period or state of inactivity or equilibrium.
And by the way, the most accepted theory for the 120,000 year long ice age cycle, is plants using up most of the CO2, that causing cooling, that kills the plants, they decompose, releasing the CO2 again, which then causes warming, that again induces high plant growth.
If we interfere with a mass plant die off, that's a bad thing?
I keep using the word "stasis" correctly.
Way back 200 million years ago, things were very chaotic and not at all cyclic, regular, or with any equilibrium.
Ice ages then were very long, like millions of years, and irregular.
Then about 3 million years ago, things changed and ice ages because very regular, cyclic, and an equilibrium was established.
I know of at least 12 regular ice ages that then happened like clockwork, all lasting about 120,000 years, but having no permanent effects.
The climate had entered into a pattern of dynamic equilibrium.
While interfering with a plant die off may not be a very bad thing, we are far from the cold point of the cycle that would freeze plants.
In fact, we are just after the warmest part of the cycle.
So then to initiate artificial additional warming, while still under the influence of the warmest part of the cycle, would essentially be doubling up and loading a new artificial warming right on top of the natural old warming.
And a double warming could be a disaster.
Worst case scenario could be a runaway race condition that gets so hot that all surface water evaporates, like it does on Venus.
The surface of the earth could become the temperature of molten lead.
The two feedback mechanisms that could cause this rapid heat acceleration are water vapor, which simply evaporates from the ocean more easily as CO2 warms the oceans, and methane released from frozen deposits on the bottoms of oceans, tundra, etc.
Methane is the most scary because is it about 20 times better at retaining planetary heat.
And there are huge deposits frozen on the ocean floor. There have been ships sunk in the Carribean Sea from being above a bubble of methane gas release from a melting of frozen methane hydrate. And Siberia, Antarctica, etc., have vast frozen swamps full of frozen methane hydrate.
I keep using the word "stasis" correctly.
Stasis means the temperature barely changes. Ice ages and then warm periods isn't stasis. Moron.
The surface of the earth could become the temperature of molten lead.
Sorry, you'll have to show me the math behind your claim.
The overall temperature does not change.
The ice age cycles have cold and hot swings, but each cycle is identical to the last, for the last 12 ice ages at least.
Only now is the question, because we are artificially initiating another warming cycle on top of the last one that is not gone yet.
Only now are we breaking the stasis of the repeating ice age cycles.
The definition of statis did not say just barely changes, but an equilibrium that has a constant fluctuation that does not change and always repeats identically.
The description of Venus said it was 3 times hotter than it should be from the sun alone, due to greenhouse gas heat retention.
Venus is 460 degrees C. That would be 153 degrees C, but that is because of the close proximity of the sun. Regardless of what the Earth could reach as its maximum, clearly we would not want to find out. A positive feedback, race condition where more retained heat cause even more greenhouse gases, such as from the oceans evaporating and frozen methane melting, would not be pleasant.