Sheldon
Senior Member
- Apr 2, 2010
- 5,213
- 1,431
- 48
.Nature News Blog: Firm launches two stem cell trials against blindness
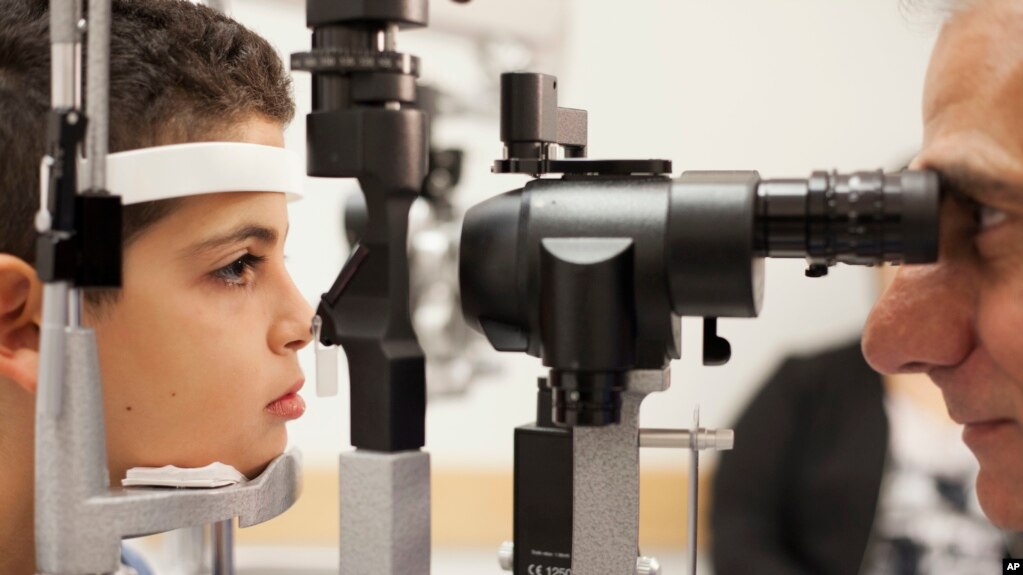
Now, the second and third hESC trials have been launched. On July 12, in an operating room at the University of California, Los Angeles, the first subject in each of the trials -- one for a rare form of blindness that usually begins in childhood, the other for a common cause of blindness in the elderly -- was treated with retinal pigment epithelial (RPE) cells derived from hESCs. Details are available in this press release from the sponsoring company, Advanced Cell Technology, based in Santa Monica.
...
The trials, each of which aims to enroll 12 patients, will assess over the course of one year the safety and tolerability of ascending dosages of the cells. The first two patients received what are considered small doses -- 50,000 cells each -- transplanted into the tissue directly under their retinas.
"Early indications are that the patients tolerated the surgical procedures well," Schwartz said in the press release.