CultureCitizen
Silver Member
- Jun 1, 2013
- 1,932
- 140
- 95
- Thread starter
- #241
Interesting.... any links ?It is possible that the CO2 doesn't have an immediat effect , but rather it takes several years to cause an increase in temperature.So by simple observation we can see the problem with the hypothesis of runaway temp caused by CO2 or MMGW. During the time they claim runway rise, it was nothing of the sort and even given the rise in CO2 there was no discernible increase in that natural rise.
So tell me, where exaxctly is mans input signal? Where is the rise attributed to man contributions? It is not in the empirically observed evidence.
I'll admit that's speculation on my part, since I am no climatologist. On the other hand to say that chart is correct you would have to proove the oposite : that the full blown effects of increase in CO2 are felt in the following year in which it was produced.
There goes a plausible explanation for the lack of correlation in your chart .
All empirical data says otherwise. The data shows warming occurs and then, between 400 and 800 years AFTER the warming there is a corresponding rise in CO2. CO2 has NEVER initiated a rise in global temperature. It has always risen as a result of warming.
Here you go....
"Carbon dioxide follows temperature in the Vostok Ice Cores
In the 1990′s the classic Vostok ice core graph showed temperature and carbon in lock step moving at the same time. It made sense to worry that carbon dioxide did influence temperature. But by 2003 new data came in and it was clear that carbon lagged behind temperature. The link was back to front. Temperatures appear to control carbon, and while it’s possible that carbon also influences temperature these ice cores don’t show much evidence of that. After temperatures rise, on average it takes 800 years before carbon starts to move. The extraordinary thing is that the lag is well accepted by climatologists, yet virtually unknown outside these circles. The fact that temperature leads is not controversial. It’s relevance is debated."
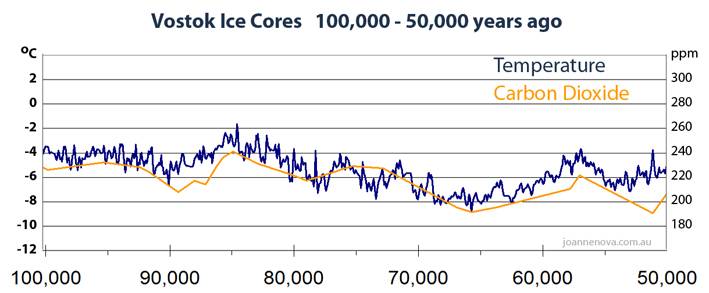
The 800 year lag in CO2 after temperature – graphed « JoNova

ANZICE - Antarctic Research Centre - Victoria University of Wellington
Interesting, thanks.
I am not sure of what conclusions to draw .
First, we are facing a situation that is new in the last 400,000 years ( at least) : carbon spiking before temperature rise.
Second it is intresting to note how after reaching a maximum both temperatures and co2 drop almost vertically.
Third , our situation is unique in another sense judging by the second chart: the changes in co2 and methane levels we are seing usually take 15,000 years . We are now having the same level of change in 100 years.
I can only conclude that the previous natural cycles have a very different nature
We can conclude that a raise in temperature creates a rise in co2 with a lag of 800 years or so. But the oposite will not necesarily be true ( a rise of co2 will create a rise of temperature with 800 years of lag ).


