Stupidity is indeed unlimited.
In the literature Fluorescence, Phosphorescence, and Chemiluminescence are all said to be thermodynamically spontaneous
They all can cause emission of photons to all objects, warmer or colder.
Have you ever seen a chemical lightstick, which you can hold in your hand, and will illuminate something too hot to touch
That is spontaneous EM energy moving from a cold substance to a warmer substance. That simple everyday device solidly refutes your claim.....
And
it takes that kind of stupidity to claim what you just claimed...which is that since photons from a lightstick which indeed are the product of a spontaneous process can radiate towards a hot object proves that raising the temperature of the hot object with a colder one
can be achieved with no effort since it must be a spontaneous process because photons can be radiated at it.
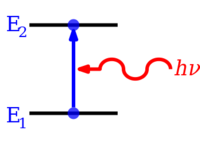
Hahaha I would like to see how a 0.1 eV photon can bump up the energy level of an electron`s orbital to 10 eV
meaning it has been absorbed and raised the temperature as a result of a spontaneous process.
All the while you and the other idiots who agree with you have been pretending to understand not just basic physics, but also quantum physics.
The latter has to be re-written from:
Electrons in atoms and molecules can change (make
transitions in) energy levels by emitting or absorbing a
photon (of
electromagnetic radiation),
whose energy must be exactly equal to the energy difference between the two levels.
To Wuwei et al:
Electrons do
not not transition to discrete energy levels but can be at any energy level and
it is no longer required that the photon`s energy is exactly the same as the energy difference as previously stated having to be E2-E1=hv. We have proven this because we could shine a lightstick at a 2500 deg Tungsten filament .