Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.

Note: This feature currently requires accessing the site using the built-in Safari browser.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Trenberth's Energy Budget
- Thread starter IanC
- Start date
wirebender
Senior Member
this is the crux of the problem. wirebender does not understand the difference between radiation and heat flow. radiation is a function of the temperature of the radiating object (in very simple terms) and heat flow is the measurement of the size and direction of net radiation.
The crux Ian, is that this is all just way to far over your head for you to discuss. When you said that the Stefan-Boltzman equations for dealing with blackbody radiation were far fetched and incorrect, you lost any remnant of credibility you might have retained.
Get a clue Ian, it isn't to late.
- Thread starter
- #63
wirebender- this is what you posted
Re: Vacuum Chamber with plates.
First, identify the ONLY energy source in the Vacuum Chamber with an electric heater.
The ONLY energy source is the ELECTRIC HEATER that heats a plate with electricity to a temperature of 150 deg F or 338.56 K.
Asuume an emissivity = 1 and a surface Area for the plate = 1 m^2
Using the Stefan-Boltzmann Law, the Watts provided by the Electric Heater is:
P = e*BC*A*T^4
Where P = net radiated power (Watts), e = emissivity, BC = Stefans constant 5.67 X 10^-8, A = area and T = temperature of radiator in K
P = (5.67X10^-8) X 1m^2 X (338.56 K)^4 = 744.95 Watts
(***Thats ALL the Energy Available and cannot be exceeded without CREATING ENERGY***)
The EM field produced by the plate is 744.95 Watts/ 1 m^2 = 744.95 w/m^2
If another identical non-heated and colder plate is inserted into the Vacuum Chamber next to the heated Plate then:
The 2nd Plate also has an emissivity = 1 and a surface Area for the 2nd plate = 1 m^2
We can easily determine the equilibrium temperature of both plates by using the Stefan-Boltzmann Law and The Law of Conservation of Energy.
The TOTAL amount of energy available is 744.95 Watts and both plates will have the same temperature at equilibrium, so they can be considered to be a single radiating body with double the radiating surface area.
The area of both plates = 2 m^2 so the Radiation emitted by both plates at equilibrium = 744.95 Watts / 2 m^2 = 372.48 w/m^2
And the equilibrium temperature for both plates will be 284.69 K or 53 deg F.
is it a different experiment by [Gord] or just his description of Spencer's example? you didnt specify.
it doesnt really matter because Spencer's description is correct and your copied description is incorrect.
you still havent described the mechanism by which you think both plates (or bars) will come to equilibrium at the same temperature even though one is heated and the other is not.
you still havent explained why you think the heat loss in the direction of the second bar is the same as the heat loss in the direction of the cooled vacuum, even though there is an obvious difference in temperatures.
the math in your example is not only wrong but farfetched. no wonder you refuse to bump your post where you 'proved that photons disappear in space, and did the math'. obviously it was just as infintile and incorrect as your 'proof' that Spencer was wrong.
I just want to address a few things directly...
"you still havent described the mechanism by which you think both plates (or bars) will come to equilibrium at the same temperature even though one is heated and the other is not."
Its due to the laws of thermodynamics Ian... Particularly the last one I mentioned in a previous post.. The zeroth law... I suggest you read it again..
here is a better explanation than I gave I believe...
Zeroth Law, Thermal Equilibrium and Temperature
Zeroth Law
The zeroth law is a consequence of thermal equilibrium and allows us to conclude that temperature is a well-defined physical quantity. The zeroth law of thermodynamics states:
If a body A and a body B are both in equilibrium with each other; then a body C which is in thermal equilibrium with body B will also be in equilibrium with body Aand the temperature of body C is equal to the temperature of body A.
It is the zeroth law, because it preceeds the first and second laws of thermodynamics and is also a tacit assumption in both laws.
We use the zeroth law when we wish to compare the temperatures of two objects, A and B. We can do this by using a thermometer, C and placing it again object A it reaches thermal equilibrium with object A and measure the temperature of A. Placing the thermometer against object B until thermal equilibrium is reached we measure the temperature of object B. If they are the same temperature then they will be in thermal equilibrium with each other.
Now if you are somehow changing the distance of the plates from one another that would be effected by that new distance. I was under the assumption the plates were placed on top of one another. This was implied throughout the experiment I believe. if they are indeed in contact with one another the outside temps only effect the outside surface areas of the plates. Of course the closer to the heat source the warmer it will be no one made any claim to the contrary. however, the assumed term "all things being equal" will apply here.
The entire experiment is a thought experiment. The physical reality is its not even a good example of how a real physical experiment would be. First there is always a difference in the temperature of any heated surface the farther it gets from its point of contact with the heat source. Second standing plates are not a moving atmosphere with all the variables it entails. Third, the vacuum is only applicable to the relation of the Sun to the atmospheric surface. Once it hits that atmosphere the vacuum is no longer a factor. The earth sits in a vacuum, but the atmosphere is not a vacuum, and that is the part that is in contention in greenhouse effect theory. That would make the entire premise of the experiment pointless.
However you brought this experiment into the discussion and professed its accuracy. So we tried to show how it was not an accurate example of how the "greenhouse effect" works. You vehemently defended it and still do so now. This experiment forces many of the natural finer points and details to be ignored.
Now if you want us to ignore the finer points and details and allow this experiment to go through to its claimed result, you cannot very well fall back on those finer points to defend it when it falls flat.. The experiment assumes "all things being equal" from the start, that is the only way it can work even in a thought experiment. So either all things are indeed equal in it or all things are as they would be in the real world, either way it falls flat. But you must stick to one or the other, switching between them only confounds the thing and makes it impossible..
I agree with the zeroith law but it does not apply to one object that is being heated and another one that is only absorbing the radiation from the first. the two objects are not the same temperature even if they are in an equilibrium of sorts.
if you are talking about Spencer's experiment the diagram specifically shows separation between the two bars, to stop conduction. and states a vacuum, to stop convection. he was specifically dealing with the radiation properties of objects. and he successfully showed that adding an object between the source of heat and the cold outside would raise the temperature of the radiating source.
an analogy is a water source (electricity) going to a connection with multiple hoses and sprinklers (the heated bar). if you put a crimp into one of the hoses (second bar which hampers the ability to radiate to the cold outside) then the water pressure backs up and the remaining sprinklers are more forceful (the heated bar is warmer). the whole thought experiment is logical and easy to understand if you just think about it.
- Thread starter
- #64
I see your test had the same result...
EDIT: Maybe next time you should test it BEFORE neg repping me and making the false claim...
I'll pos rep you when I am able. again, my apologies
- Thread starter
- #65
gslack said-
Spencer's experiment is not an explanation of the greenhouse effect. it is an explanation of why the greenhouse effect is not in violation of thermodynamic laws, specifically 'a cooler body cant warm a warmer body'.
However you brought this experiment into the discussion and professed its accuracy. So we tried to show how it was not an accurate example of how the "greenhouse effect" works. You vehemently defended it and still do so now. This experiment forces many of the natural finer points and details to be ignored.
Spencer's experiment is not an explanation of the greenhouse effect. it is an explanation of why the greenhouse effect is not in violation of thermodynamic laws, specifically 'a cooler body cant warm a warmer body'.
wirebender
Senior Member
I agree with the zeroith law but it does not apply to one object that is being heated and another one that is only absorbing the radiation from the first. the two objects are not the same temperature even if they are in an equilibrium of sorts.
Mighty caucasian of you even though you clearly don't grasp its inplications.
he was specifically dealing with the radiation properties of objects. and he successfully showed that adding an object between the source of heat and the cold outside would raise the temperature of the radiating source.
The only thing spencer showed is that he doesn't recognize a heat sink when he imagines it.
an analogy is a water source (electricity) going to a connection with multiple hoses and sprinklers (the heated bar). if you put a crimp into one of the hoses (second bar which hampers the ability to radiate to the cold outside) then the water pressure backs up and the remaining sprinklers are more forceful (the heated bar is warmer). the whole thought experiment is logical and easy to understand if you just think about it.
The math is logical and easy to understand, your interpretation of the experiment and end result is twisted in tangled up knots.
wirebender
Senior Member
Spencer's experiment is not an explanation of the greenhouse effect. it is an explanation of why the greenhouse effect is not in violation of thermodynamic laws, specifically 'a cooler body cant warm a warmer body'.
The experiment was a failure precisely because it violates both the 2nd law of thermodynamics and the law of conservation of energy. You are hopeless Ian.
- Thread starter
- #68
I agree with the zeroith law but it does not apply to one object that is being heated and another one that is only absorbing the radiation from the first. the two objects are not the same temperature even if they are in an equilibrium of sorts.
Mighty caucasian of you even though you clearly don't grasp its inplications.
he was specifically dealing with the radiation properties of objects. and he successfully showed that adding an object between the source of heat and the cold outside would raise the temperature of the radiating source.
The only thing spencer showed is that he doesn't recognize a heat sink when he imagines it.
an analogy is a water source (electricity) going to a connection with multiple hoses and sprinklers (the heated bar). if you put a crimp into one of the hoses (second bar which hampers the ability to radiate to the cold outside) then the water pressure backs up and the remaining sprinklers are more forceful (the heated bar is warmer). the whole thought experiment is logical and easy to understand if you just think about it.
The math is logical and easy to understand, your interpretation of the experiment and end result is twisted in tangled up knots.
from a description of heat sinks-
The heat transfer from the heatsink is mediated by two effects: convection via the coolant, and thermal radiation.
Heat transfer by radiation is a function of both the heat sink temperature, and the temperature of the surroundings that the heat sink is optically coupled with. When both of these temperatures are on the order of 0 °C to 100 °C, the contribution of radiation compared to convection is generally small, and this factor is often neglected. In this case, finned heat sinks operating in either natural-convection or forced-flow will not be effected significantly by surface emissivity.
In situations where convection is low, such as a flat non-finned panel with low airflow, radiative cooling can be a significant factor. Here the surface properties may be an important design factor. Matte-black surfaces will radiate much more efficiently than shiny bare metal.[10] A shiny metal surface has low emissivity, so it absorbs and radiates only a small amount of radiant heat, while matte-black has high emissivity so it absorbs and radiates radiant heat highly. The emissivity in the visible spectrum is closely related to color. For most materials, the emissivity in the visible spectrum is similar to the emissivity in the infrared spectrum; however there are exceptions, notably certain metal oxides that are used as "selective surfaces".
In a vacuum or in outer space, there is no convective heat transfer, thus in these environments, radiation is the only factor governing heat flow between the heat sink and the environment. For a satellite in space, a 100 °C (473 Kelvin) surface facing the sun will absorb a lot of radiant heat, since the sun's surface temperature is nearly 6000 Kelvin, whereas the same surface facing deep-space will radiate a lot of heat, since deep-space has an effective temperature of only a few Kelvin.
please explain to me how you think the two bars will equilibrate to the same temperature in Spencer's experiment even though one is heated and the other is not.
you have said many foolish things in the past but that is right up there with saying that a blanket make you colder.
gslack
Senior Member
- Mar 26, 2010
- 4,527
- 356
- 48
wirebender- this is what you posted
is it a different experiment by [Gord] or just his description of Spencer's example? you didnt specify.
it doesnt really matter because Spencer's description is correct and your copied description is incorrect.
you still havent described the mechanism by which you think both plates (or bars) will come to equilibrium at the same temperature even though one is heated and the other is not.
you still havent explained why you think the heat loss in the direction of the second bar is the same as the heat loss in the direction of the cooled vacuum, even though there is an obvious difference in temperatures.
the math in your example is not only wrong but farfetched. no wonder you refuse to bump your post where you 'proved that photons disappear in space, and did the math'. obviously it was just as infintile and incorrect as your 'proof' that Spencer was wrong.
I just want to address a few things directly...
"you still havent described the mechanism by which you think both plates (or bars) will come to equilibrium at the same temperature even though one is heated and the other is not."
Its due to the laws of thermodynamics Ian... Particularly the last one I mentioned in a previous post.. The zeroth law... I suggest you read it again..
here is a better explanation than I gave I believe...
Zeroth Law, Thermal Equilibrium and Temperature
Zeroth Law
The zeroth law is a consequence of thermal equilibrium and allows us to conclude that temperature is a well-defined physical quantity. The zeroth law of thermodynamics states:
If a body A and a body B are both in equilibrium with each other; then a body C which is in thermal equilibrium with body B will also be in equilibrium with body Aand the temperature of body C is equal to the temperature of body A.
It is the zeroth law, because it preceeds the first and second laws of thermodynamics and is also a tacit assumption in both laws.
We use the zeroth law when we wish to compare the temperatures of two objects, A and B. We can do this by using a thermometer, C and placing it again object A it reaches thermal equilibrium with object A and measure the temperature of A. Placing the thermometer against object B until thermal equilibrium is reached we measure the temperature of object B. If they are the same temperature then they will be in thermal equilibrium with each other.
Now if you are somehow changing the distance of the plates from one another that would be effected by that new distance. I was under the assumption the plates were placed on top of one another. This was implied throughout the experiment I believe. if they are indeed in contact with one another the outside temps only effect the outside surface areas of the plates. Of course the closer to the heat source the warmer it will be no one made any claim to the contrary. however, the assumed term "all things being equal" will apply here.
The entire experiment is a thought experiment. The physical reality is its not even a good example of how a real physical experiment would be. First there is always a difference in the temperature of any heated surface the farther it gets from its point of contact with the heat source. Second standing plates are not a moving atmosphere with all the variables it entails. Third, the vacuum is only applicable to the relation of the Sun to the atmospheric surface. Once it hits that atmosphere the vacuum is no longer a factor. The earth sits in a vacuum, but the atmosphere is not a vacuum, and that is the part that is in contention in greenhouse effect theory. That would make the entire premise of the experiment pointless.
However you brought this experiment into the discussion and professed its accuracy. So we tried to show how it was not an accurate example of how the "greenhouse effect" works. You vehemently defended it and still do so now. This experiment forces many of the natural finer points and details to be ignored.
Now if you want us to ignore the finer points and details and allow this experiment to go through to its claimed result, you cannot very well fall back on those finer points to defend it when it falls flat.. The experiment assumes "all things being equal" from the start, that is the only way it can work even in a thought experiment. So either all things are indeed equal in it or all things are as they would be in the real world, either way it falls flat. But you must stick to one or the other, switching between them only confounds the thing and makes it impossible..
I agree with the zeroith law but it does not apply to one object that is being heated and another one that is only absorbing the radiation from the first. the two objects are not the same temperature even if they are in an equilibrium of sorts.
if you are talking about Spencer's experiment the diagram specifically shows separation between the two bars, to stop conduction. and states a vacuum, to stop convection. he was specifically dealing with the radiation properties of objects. and he successfully showed that adding an object between the source of heat and the cold outside would raise the temperature of the radiating source.
an analogy is a water source (electricity) going to a connection with multiple hoses and sprinklers (the heated bar). if you put a crimp into one of the hoses (second bar which hampers the ability to radiate to the cold outside) then the water pressure backs up and the remaining sprinklers are more forceful (the heated bar is warmer). the whole thought experiment is logical and easy to understand if you just think about it.
Well I am glad you agree with the zeroth law.. With it being a natural law you really little choice in the matter it simply is regardless of your agreement with it....

Ian will you PLEASE stop with the nonsense about "if you are talking about spencer's experiment" ... There wouldn't be either experiment in this discussion if you hadn't brought them here.. Dude pick one already! Your first one or your second one matters little to me but PICK ONE! You brought them both into the discussion for pete's sake...
Now after you said all that about which experiment, you went and did this again...
"an analogy is a water source (electricity) going to a connection with multiple hoses and sprinklers (the heated bar). if you put a crimp into one of the hoses (second bar which hampers the ability to radiate to the cold outside) then the water pressure backs up and the remaining sprinklers are more forceful (the heated bar is warmer). the whole thought experiment is logical and easy to understand if you just think about it."
Ian WTH??? You just brought in another experiment using a completely different set of variables and using completely different methodology.. My god man are you aware that you have been asking us which experiment we are talking about in each post, when you were the one who brought all of them in? look if you can't focus on one why bring other ones in? YOU confounded this conversation just like you have done in the previous ones.. you did it because you cannot make the claims hold water, yet you feel you must..
I am sorry Ian... The theory has holes. its a fact. Scientists are human first. They can and do make mistakes, and with their brilliance and egos its very difficult to get many of them to admit them especially when they are this big.. They will do what they have to, to protect their pride, position and esteem, as well their research they may have spent their lives on. Not to mention colleges that support them, and students hoping to go into the same field one day. That is an insane amount of pressure to be not only right about this, but insure the science and investments in it...
Now your new experiment above....
Dude electricity, is not heat... And water is a liquid. Come on Ian you damn well know better.... that was just a bunch unrelated nonsense to further confound the discussion.. Are you really wanting to prove something here or just wanting to give the appearance you proved something here?
EDIT: sorry forgot one thing..
You said... "the diagram specifically shows separation between the two bars, to stop conduction. and states a vacuum, to stop convection. he was specifically dealing with the radiation properties of objects. and he successfully showed that adding an object between the source of heat and the cold outside would raise the temperature of the radiating source."
Ian first and foremost, the experiment was in fact an effort by spencer to show how a cold object can make a hot object hotter in some circumstances, its entire point was to show how this can happen in the case of greenhouse effect in the atmosphere...
Now please remember that first part especially.. I will state it again...."the experiment was in fact an effort by spencer to show how a cold object can make a hot object hotter in some circumstances" Okay that was what spencer made a point of early in that article you cited.....
Now in spencers experiment he mentions a plate and an electric heater, then he mentions another plate but his picture shows a bar... See the problems yet? Later on he mentions bars, then plates, and comments on them back and forth making the entire thing very hard to follow fro a true skeptical mind... if you go in there with the preconceived notion you are going to get a logical experiment from a solid scientist with no ambiguity or deception, you will more times than not accept what he shows as true and accurate. However clear your mind and treat him and his post as that of a person with the same strengths and weaknesses as any other human, and look it over with the desire to know the truth whatever it may be, you will end up with questions that the experiment cannot address..
I ask you once more to go and read it through very carefully, as will I... When I am done I will come clean with you.. If I find hes right I will say so... But I ask you to do the same and without all the confounding and nonsense.. No more double talk, no more BS obfuscation just flat out let the chips fall where they may truth, no matter who gets their pride hurt or what we find... Agreed?
I hope so, either way I am going to have to switch to my laptop for a bit and do some work, Then I will go over that experiment with all my natural anal-retentiveness and either show how it is wrong or how it is right to the best of my ability..
Last edited:
- Thread starter
- #70
gslack- I linked Spencer's article, then wirebender said it was debunked by some poster named Gord. wire then put up a comment about plates instead of bars that had bogus math. it may or may not be about Spencer's thought experiment but you guys werent explicit even when I asked for clarification. if it was about Spencer then it was hopelessly flawed.
so far I have tried to explain the actual physical characteristics of the experiment. when that failed I gave you a simple analogy that anyone older than eight could understand.
I have tried in many ways to point out where you and wirebender are confused but all you do is repeat that I am wrong with no counter explanation nor any response to my explicit simple questions.
for instance, I would be very interested in hearing why you think a heated object will be the same temperature as an unheated object when both are losing heat to a cooled vacuum container. will you at least attempt to answer that question which is the basis of your supposed debunking of Spencer?
so far I have tried to explain the actual physical characteristics of the experiment. when that failed I gave you a simple analogy that anyone older than eight could understand.
I have tried in many ways to point out where you and wirebender are confused but all you do is repeat that I am wrong with no counter explanation nor any response to my explicit simple questions.
for instance, I would be very interested in hearing why you think a heated object will be the same temperature as an unheated object when both are losing heat to a cooled vacuum container. will you at least attempt to answer that question which is the basis of your supposed debunking of Spencer?
gslack
Senior Member
- Mar 26, 2010
- 4,527
- 356
- 48
Okay I am back.. this is going to take a bit so stay the course...
Forget plates, it only confounds his experiment and confuses the point.. Stick with bars...
Okay he says a cooling a cylindrical cooling chamber (he says round but I am sure he meant cylindrical) and he puts a bar in it that is being heated by an electric heater. In this case lets say the bar itself is a heating element as I feel it was implied from the picture. he goes on to say the chamber is in a vacuum and cooled. he does not specify the temperature it is cooled to. And that bar is heated to a constant temperature of 150 degrees F... Okay first part done.. we following each other here?
Then he adds a second bar next to the first, he does not specify exact distance. And contends the second bar through radiation from the first bar will warm up to a temp he chose of 100 F (equilibrium). he then contends the introduction of that second bar will cause the first heated bar to get even hotter... Agreed? okay..
Problems...
1. First and foremost spencer calls the bars plates, and then bars repeatedly especially after he introduces the second one. Perhaps this was an error, but the fact is it makes the experiment harder to follow and as a scientist he should know better.. Proper, established terms for respective elements in an experiment even a thought experiment is paramount.. Its enough to get the entire thing dismissed off-hand on any serious scientific level..
2. He does not give a temperature for the cooling or anything that would give an idea of it. he also fails to mention a material for the chamber. These two factors alone make the experiment dubious, but added to the already mentioned problems above, it only furthers the pointlessness of it all...
3. He mentions the second bar acts as an insulator and slows the heat loss of the first bar causing it to get hotter. This may seem plausible but he fails to realize one simple fact.. the second bar is not in equilibrium with the first bars temp. bar #1 150 F, bar #2 100 F...its in equilibrium with the amount of radiative energy received from the first bar comparative to distance, and whatever the temperature of the cooled chamber is and the various energy transfer rates the conditions allow. Again we do not know this as he did not tell us materials used in the chamber or the temp it is cooled to.. If Place my hand next a heat source the closer I get the warmer it will be, and if its colder out the closer i can get to it before I get burned. So there is a difference relative to distance and temperature regardless of a vacuum or atmosphere. This again makes the experiment a failure...
4. he makes the claim several times in the experiment as well as responses to posters, that because the first bar is constantly receiving new energy from the wires and constantly radiating new heat, then so is the second bar and that allows the second law of thermodynamics to be a non-factor or however he tried to explain it (he gets very vague on that and says it doesn't violate the rule when it clearly does). Now we both know that either he breaks the law with this or he doesn't. Breaking it and claiming he doesn't is just retarded.. the experiment breaks the law period.. if the amount of energy it takes to make the first bar 150 F is a constant flow of new energy, than that is what it takes to make that bar 150F. And the amount of radiative energy from that bar it takes to make the second bar 100F is again that amount constantly. OF course its new energy it makes no difference the amount of heat generated from that energy will still be 150F, new or old, or stored or whatever, the point is the heat it gives off. the second law tells us energy flows in one direction, and that direction is from the source outward. So long as the second bars temp is below that of the first that energy flow will go from the hotter to the colder period no ifs ands or buts about it. if there is any incidental extra warming its due to proximity and reflection.
5. he makes it very clear that this experiment was to show how this type colder to warmer reaction can work in our atmosphere. That is shown from the beginning and at the end. So yes it is an attempt to show how one aspect of greenhouse effect works. Using completely unrelated and unrealistic means and methods...
Basically his contention falls flat on many levels but the one that bothers me the most is the fact he says one thing then shows another, claims one thing and shows another..
That and the very simple factor that the heat source is the first bar which would represent the sun. meaning the second would have to represent the earth. So does our presence make the sun hotter? Don't think so, and that is the entire point... Nothing in his experiment has an electro-magnetic field, nothing orbits around the heat source, there is nothing representing the atmosphere, there is no telling what he cools the chamber too or the materials used.
its nonsense designed to confound a simple thing and I am now officially disgusted with Spencer... From reading his responses I see very clearly is not a scientist as much as he is an egotist...
Solid objects are not gases... The Stefan-Boltzmann Law was not written for gases, and he relies on its principles to make his experiment and apply it to greenhouse theory.. Spencer is desperate to ride his gravy train till he can't any longer...
Earth's atmosphere may be more efficient at releasing energy to space than climate models indicate, satellite data suggest
"Earth's Atmosphere May Be More Efficient at Releasing Energy to Space Than Climate Models Indicate, Satellite Data Suggest
ScienceDaily (July 29, 2011) Data from NASA's Terra satellite suggests that when the climate warms, Earth's atmosphere is apparently more efficient at releasing energy to space than models used to forecast climate change may indicate, according to a new study."
Spencer's own words..""The satellite observations suggest there is much more energy lost to space during and after warming than the climate models show," Spencer said. "There is a huge discrepancy between the data and the forecasts that is especially big over the oceans."
Yeah hes a sell out... he claims one thing but then when its found to be inaccurate, he doesn't come out and say so anywhere he doesn't have to... on his blog its business as usual after all he has books to sell and money to make. But in the science sites very few people read it's another story...
Forget plates, it only confounds his experiment and confuses the point.. Stick with bars...
Okay he says a cooling a cylindrical cooling chamber (he says round but I am sure he meant cylindrical) and he puts a bar in it that is being heated by an electric heater. In this case lets say the bar itself is a heating element as I feel it was implied from the picture. he goes on to say the chamber is in a vacuum and cooled. he does not specify the temperature it is cooled to. And that bar is heated to a constant temperature of 150 degrees F... Okay first part done.. we following each other here?
Then he adds a second bar next to the first, he does not specify exact distance. And contends the second bar through radiation from the first bar will warm up to a temp he chose of 100 F (equilibrium). he then contends the introduction of that second bar will cause the first heated bar to get even hotter... Agreed? okay..
Problems...
1. First and foremost spencer calls the bars plates, and then bars repeatedly especially after he introduces the second one. Perhaps this was an error, but the fact is it makes the experiment harder to follow and as a scientist he should know better.. Proper, established terms for respective elements in an experiment even a thought experiment is paramount.. Its enough to get the entire thing dismissed off-hand on any serious scientific level..
2. He does not give a temperature for the cooling or anything that would give an idea of it. he also fails to mention a material for the chamber. These two factors alone make the experiment dubious, but added to the already mentioned problems above, it only furthers the pointlessness of it all...
3. He mentions the second bar acts as an insulator and slows the heat loss of the first bar causing it to get hotter. This may seem plausible but he fails to realize one simple fact.. the second bar is not in equilibrium with the first bars temp. bar #1 150 F, bar #2 100 F...its in equilibrium with the amount of radiative energy received from the first bar comparative to distance, and whatever the temperature of the cooled chamber is and the various energy transfer rates the conditions allow. Again we do not know this as he did not tell us materials used in the chamber or the temp it is cooled to.. If Place my hand next a heat source the closer I get the warmer it will be, and if its colder out the closer i can get to it before I get burned. So there is a difference relative to distance and temperature regardless of a vacuum or atmosphere. This again makes the experiment a failure...
4. he makes the claim several times in the experiment as well as responses to posters, that because the first bar is constantly receiving new energy from the wires and constantly radiating new heat, then so is the second bar and that allows the second law of thermodynamics to be a non-factor or however he tried to explain it (he gets very vague on that and says it doesn't violate the rule when it clearly does). Now we both know that either he breaks the law with this or he doesn't. Breaking it and claiming he doesn't is just retarded.. the experiment breaks the law period.. if the amount of energy it takes to make the first bar 150 F is a constant flow of new energy, than that is what it takes to make that bar 150F. And the amount of radiative energy from that bar it takes to make the second bar 100F is again that amount constantly. OF course its new energy it makes no difference the amount of heat generated from that energy will still be 150F, new or old, or stored or whatever, the point is the heat it gives off. the second law tells us energy flows in one direction, and that direction is from the source outward. So long as the second bars temp is below that of the first that energy flow will go from the hotter to the colder period no ifs ands or buts about it. if there is any incidental extra warming its due to proximity and reflection.
5. he makes it very clear that this experiment was to show how this type colder to warmer reaction can work in our atmosphere. That is shown from the beginning and at the end. So yes it is an attempt to show how one aspect of greenhouse effect works. Using completely unrelated and unrealistic means and methods...
Basically his contention falls flat on many levels but the one that bothers me the most is the fact he says one thing then shows another, claims one thing and shows another..
That and the very simple factor that the heat source is the first bar which would represent the sun. meaning the second would have to represent the earth. So does our presence make the sun hotter? Don't think so, and that is the entire point... Nothing in his experiment has an electro-magnetic field, nothing orbits around the heat source, there is nothing representing the atmosphere, there is no telling what he cools the chamber too or the materials used.
its nonsense designed to confound a simple thing and I am now officially disgusted with Spencer... From reading his responses I see very clearly is not a scientist as much as he is an egotist...
Solid objects are not gases... The Stefan-Boltzmann Law was not written for gases, and he relies on its principles to make his experiment and apply it to greenhouse theory.. Spencer is desperate to ride his gravy train till he can't any longer...
Earth's atmosphere may be more efficient at releasing energy to space than climate models indicate, satellite data suggest
"Earth's Atmosphere May Be More Efficient at Releasing Energy to Space Than Climate Models Indicate, Satellite Data Suggest
ScienceDaily (July 29, 2011) Data from NASA's Terra satellite suggests that when the climate warms, Earth's atmosphere is apparently more efficient at releasing energy to space than models used to forecast climate change may indicate, according to a new study."
Spencer's own words..""The satellite observations suggest there is much more energy lost to space during and after warming than the climate models show," Spencer said. "There is a huge discrepancy between the data and the forecasts that is especially big over the oceans."
Yeah hes a sell out... he claims one thing but then when its found to be inaccurate, he doesn't come out and say so anywhere he doesn't have to... on his blog its business as usual after all he has books to sell and money to make. But in the science sites very few people read it's another story...
wirebender
Senior Member
please explain to me how you think the two bars will equilibrate to the same temperature in Spencer's experiment even though one is heated and the other is not.
Because they are in a vacuum Ian where conduction and convection into the atmosphere are eliminated. Radiation is the only means of bleeding off heat and the bars (plates;same thing) are in close proximity. They will achieve equilibrium or very close to it.
As I have already told you, volumes have been written about the issue of heat within vacuum tubes from the old days when everything used them. In a vacuum, materials heated and passive reach an equilibrium temperature very quickly. Great pains had to be taken in the design of vacuum tube components using materials of widely varied absorptivity and emissivity, and wild heat sink designs were fabricated precisely to keep the internal components of the vacuum tube from reaching thermal equilibrium. It doesn't happen in the open atmosphere because convection and conduction are in operation to carry energy away from the heat sink. Not so in a vacuum.
Face it Ian, math and the laws of physics are stronger than your faith in a mythical greenhouse effect.
The math is correct and is a correct useage of the Stefan-Boltzman law dealing with blackbody radiation. You are accepting your intuition over hard mathematical evidence that has been proven reliable for over a hundred years.
What else need be said?
you have said many foolish things in the past but that is right up there with saying that a blanket make you colder.
Funny you should mention that Ian, and state it with your usual confidence as if it were a statement of fact. Sorry Ian, the second law of thermodynamics defeats you again. The fact is, that a blanket does reduce your surface temeprature. Once again, your intuition, belief, faith, or whatever intermal system your intellect runs on, has lead you off in the wrong direction. The fact is, Ian, that putting a blanket over your body does make you colder just as the second law of thermodynamics predicts.
Your problem Ian, is that you believe you are smarter than the laws of physics and the math that proves those laws. You aren't.
"Human Body Emission
As all matter, the human body radiates some of a person's energy away as infrared light.
The net power radiated is the difference between the power emitted and the power absorbed:
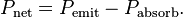
Applying the Stefan Boltzman Law
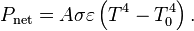
The total surface area of an adult is about 2 m², and the mid- and far-infrared emissivity of skin and most clothing is near unity, as it is for most nonmetallic surfaces.[ Skin temperature is about 33 °C, but clothing reduces the surface temperature to about 28 °C when the ambient temperature is 20 °C. Hence, the net radiative heat loss is about
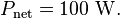
If you put a 20C blanket (which is colder) on a warmer 33C body, the surface temperature is going to reduce to about 28C. Heat flowed from the warmer body to the cooler blanket just as the 2nd law of thermodynamics predicts.
It is true that the blanket will trap warm air between the body and itself, but that heat will not increase the temperature of the body.
This buisness is a matter of the laws of physics and the mathematics that prove them Ian. Not, as you seem to believe, a matter of faith. You pit yourself against the laws of nature and you will lose every time.
wirebender
Senior Member
Well I am glad you agree with the zeroth law.. With it being a natural law you really little choice in the matter it simply is regardless of your agreement with it....
As with all the other laws of physics that he so blythely disregards in favor of his intuition, or faith, or hope, or fealty or whatever failed intellectual method he employs. (is shuck and jive considered to be an intellectual method?) I don't know about you, but I am about finished with him. The math that has been presented stands unassailed by any proof to the contrary and my bet is that the math I just gave him regarding the fact that a blanket does in fact cool you down will remain unchallenged by anything more than a verbal objection and perhaps an ad hominim delivered in frustration; and the laws of physics remain standing, unscathed. That being the case, I must assume that our side has prevailed. Congratulations. (high five, knucle bump,..... whatever)
More patience than he deserves has been lavished upon him in an effort to explain what is actually going on and the failures of trenberth's energy budget, but as they say, you can lead a horse to water but you can't make him cook cornbread once he is finished drinking.
Last edited:
- Thread starter
- #74
please explain to me how you think the two bars will equilibrate to the same temperature in Spencer's experiment even though one is heated and the other is not.
Because they are in a vacuum Ian where conduction and convection into the atmosphere are eliminated. Radiation is the only means of bleeding off heat and the bars (plates;same thing) are in close proximity. They will achieve equilibrium or very close to it.
As I have already told you, volumes have been written about the issue of heat within vacuum tubes from the old days when everything used them. In a vacuum, materials heated and passive reach an equilibrium temperature very quickly. Great pains had to be taken in the design of vacuum tube components using materials of widely varied absorptivity and emissivity, and wild heat sink designs were fabricated precisely to keep the internal components of the vacuum tube from reaching thermal equilibrium. It doesn't happen in the open atmosphere because convection and conduction are in operation to carry energy away from the heat sink. Not so in a vacuum.
Face it Ian, math and the laws of physics are stronger than your faith in a mythical greenhouse effect.
The math is correct and is a correct useage of the Stefan-Boltzman law dealing with blackbody radiation. You are accepting your intuition over hard mathematical evidence that has been proven reliable for over a hundred years.
What else need be said?
you have said many foolish things in the past but that is right up there with saying that a blanket make you colder.
Funny you should mention that Ian, and state it with your usual confidence as if it were a statement of fact. Sorry Ian, the second law of thermodynamics defeats you again. The fact is, that a blanket does reduce your surface temeprature. Once again, your intuition, belief, faith, or whatever intermal system your intellect runs on, has lead you off in the wrong direction. The fact is, Ian, that putting a blanket over your body does make you colder just as the second law of thermodynamics predicts.
Your problem Ian, is that you believe you are smarter than the laws of physics and the math that proves those laws. You aren't.
"Human Body Emission
As all matter, the human body radiates some of a person's energy away as infrared light.
The net power radiated is the difference between the power emitted and the power absorbed:
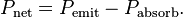
Applying the Stefan Boltzman Law
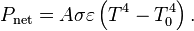
The total surface area of an adult is about 2 m², and the mid- and far-infrared emissivity of skin and most clothing is near unity, as it is for most nonmetallic surfaces.[ Skin temperature is about 33 °C, but clothing reduces the surface temperature to about 28 °C when the ambient temperature is 20 °C. Hence, the net radiative heat loss is about
"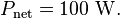
If you put a 20C blanket (which is colder) on a warmer 33C body, the surface temperature is going to reduce to about 28C. Heat flowed from the warmer body to the cooler blanket just as the 2nd law of thermodynamics predicts.
It is true that the blanket will trap warm air between the body and itself, but that heat will not increase the temperature of the body.
This buisness is a matter of the laws of physics and the mathematics that prove them Ian. Not, as you seem to believe, a matter of faith. You pit yourself against the laws of nature and you will lose every time.
wirebender- your link supports my position! skin radiates more when it is exposed. when an insulator is put between skin and a colder ambient temperature there is less heat loss. surely you didnt think that the skin underneath the clothing has cooled? are you really that stupid?
your link even spells it out. net power radiated equals power emitted less power absorbed. but there wasnt the faintest hint of photons magically being extinguished.
again. please explain how a heated object and an unheated object will equilibrate at the same temperature when the heated object is the only source of heat in an open system. just describe the heat flow from one to the other so we can understand this amazingly counterintuative feat of physics.
gslack- I havent had time to go over S's article but I will.
wirebender
Senior Member
wirebender- your link supports my position! skin radiates more when it is exposed. when an insulator is put between skin and a colder ambient temperature there is less heat loss. surely you didnt think that the skin underneath the clothing has cooled? are you really that stupid?
Of course the skin underneath the clothing has cooled Ian. The math is right there. Skin temperature drops from 33 degrees C to 28 degrees C just as the second law of thermodynamics predicts. Geez Ian, you get dumber every time you post. Which part of this exactly is it so difficult for you to grasp:
Skin temperature is about 33 °C, but clothing reduces the surface temperature to about 28 °C when the ambient temperature is 20 °C.
The skin gets cooler exactly as the 2nd law of thermodynamics predicts. Your instincts are wrong Ian and clearly you don't grasp the math so I suppose you are doomed to be wrong every time you engage the topic.
again. please explain how a heated object and an unheated object will equilibrate at the same temperature when the heated object is the only source of heat in an open system. just describe the heat flow from one to the other so we can understand this amazingly counterintuative feat of physics.
It has been explained to you over and over Ian and the math has been done. You just aren't bright enough to grasp the explanation much less grasp the math.
wirebender
Senior Member
... my bet is that the math I just gave him regarding the fact that a blanket does in fact cool you down will remain unchallenged by anything more than a verbal objection and perhaps an ad hominim delivered in frustration;
And Ian's response is....drumroll........
IanC said:surely you didnt think that the skin underneath the clothing has cooled? are you really that stupid?
Ding ding ding ding.....we have a winner!!! Once more, when the math is staring him right in the face, he doesn't get it.
- Thread starter
- #77
wirebender you have mixed apples with oranges again. the skin under clothing is NOT the surface. dont you ever actually think about the physical processes going on? the skin under the clothing is warmer than exposed skin, that is the main point in wearing them. if clothing made people colder no one in temperate or cold climates would go to the bother.
if you still think clothes or blankets make you colder please explain the mechanism of how an insulator increases heat flow rather than reducing it.
if you still think clothes or blankets make you colder please explain the mechanism of how an insulator increases heat flow rather than reducing it.
gslack
Senior Member
- Mar 26, 2010
- 4,527
- 356
- 48
Ian, the skin is the radiator, the clothing or blanket if it is colder than the skin at the time it is placed in contact with it will indeed make the skin overall cooler for a time. Once the the inside of the blanket reaches equilibrium with the skin its in contact with, then the situation will change of course. But at the point a cooler body (blanket) first makes contact with a warmer body (skin) the skin will get cooler until equilibrium is reached.
I think Wirebender is referring to that, and you are most likely referring to after equilibrium is reached. When broken down fully you will find they are two different situations both mathematically and naturally...
A good example, is a cold pillow.. I like to turn my pillow over at night so I have a cooler side on my face until I fall asleep. I can feel it making my skin cooler at first, then once equilibrium is established, it of course causes my skin to get warmer on the side touching that same pillow. I turn that same pillow over and its cold on that side, and again it cools my skin for a time...
I think Wirebender is referring to that, and you are most likely referring to after equilibrium is reached. When broken down fully you will find they are two different situations both mathematically and naturally...
A good example, is a cold pillow.. I like to turn my pillow over at night so I have a cooler side on my face until I fall asleep. I can feel it making my skin cooler at first, then once equilibrium is established, it of course causes my skin to get warmer on the side touching that same pillow. I turn that same pillow over and its cold on that side, and again it cools my skin for a time...
Last edited:
- Thread starter
- #79
Ian, the skin is the radiator, the clothing or blanket if it is colder than the skin at the time it is placed in contact with it will indeed make the skin overall cooler for a time. Once the the inside of the blanket reaches equilibrium with the skin its in contact with, then the situation will change of course. But at the point a cooler body (blanket) first makes contact with a warmer body (skin) the skin will get cooler until equilibrium is reached.
I think Wirebender is referring to that, and you are most likely referring to after equilibrium is reached. When broken down fully you will find they are two different situations both mathematically and naturally...
A good example, is a cold pillow.. I like to turn my pillow over at night so I have a cooler side on my face until I fall asleep. I can feel it making my skin cooler at first, then once equilibrium is established, it of course causes my skin to get warmer on the side touching that same pillow. I turn that same pillow over and its cold on that side, and again it cools my skin for a time...
certainly I can agree with that point. but the cool side of the pillow is mostly a combination of conductance and heat sink. I thought we were discussing radiation at equilibrium. you may even be correct at the boundary of skin and clothes because of the better thermal capacity of conduction over radiation. but a clothed body loses less heat than an uncovered one so the body will not need to burn as much food to keep at its desired temperature of 37C internally.
- Thread starter
- #80
gslack- go back and reread my description of the two bars together. there would be a temperature gradient because the heater was offset. once the bars were separated the gradient would be greatly increased because of the loss of conductance. there is no way the seperate bars would be the same temp, and there is no way that the originally closed faces would send all or even most of their radiation to the cold exterior.
Similar threads
- Replies
- 32
- Views
- 663
Latest Discussions
- Replies
- 660
- Views
- 6K
- Replies
- 32
- Views
- 78
- Replies
- 563
- Views
- 2K
- Replies
- 9
- Views
- 75
- Replies
- 1K
- Views
- 15K
Forum List
-
-
-
-
-
Political Satire 8045
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
ObamaCare 781
-
-
-
-
-
-
-
-
-
-
-
Member Usernotes 469
-
-
-
-
-
-
-
-
-
-