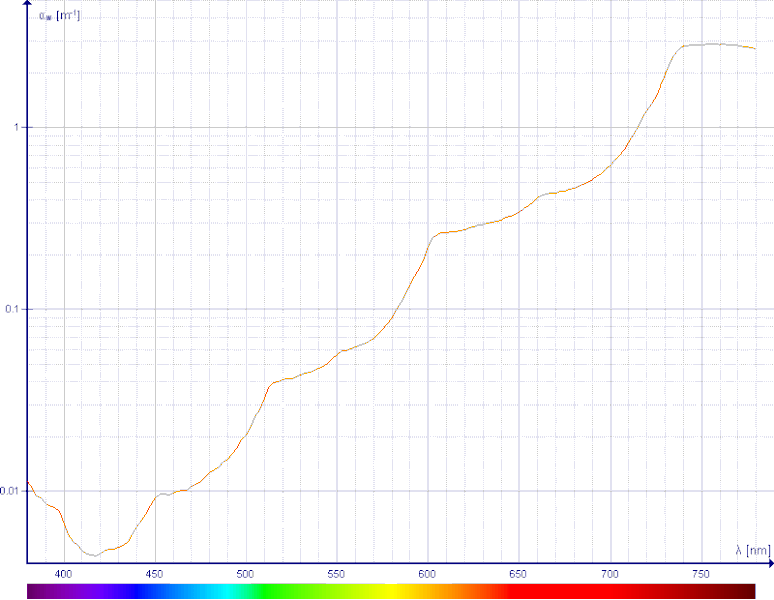
From Spencer's blog -
I frequently see the assertion made that infrared (IR) radiation cannot warm a water body because IR only affects the skin (microns of water depth), whereas solar radiation is absorbed over water depths of meters to tens of meters.
Before discussing the issue, though, we first must agree that temperature and temperature change of a body is related to rates of energy gain and energy loss by that body. If we cannot agree on the basic concept that temperature changes when energy gain does not equal energy loss, then there is no basis for further discussion.
If the surface of a water body is emitting IR, then IR must be part of its energy budget, and therefore of its temperature. Evaporation only occurs at the skin, and we know that evaporation is the major component of heat loss by water bodies. How is it that evaporation can perform this function, and IR cannot?
The temperature of land clearly is affected by IR, and that only occurs at the surface of the soil. So, how can IR affect land temperature and not ocean temperature?
If you claim that any additional IR (say, due to increasing carbon dioxide) is immediately lost by the water body through evaporation, how exactly does that occur? The surface doesnt know why it has the temperature it does, it will evaporate water based (partly) on surface temperature, and it does not distinguish where the heat comes from (solar radiation from above, mixing from below, IR from above, sensible heat flux across the air/water interface). To claim that any energy gain from IR is immediately lost by evaporation is just an assertion.
NEVERTHELESS
It might well be that solar radiation is more efficient (on a Watt per Watt basis) than IR radiation at changing ocean temperature. In other words, that IR warming of a water body is more likely to be lost through evaporation, since its warming effect does occur only at the surface, and so that energy is more likely to be lost through evaporation than absorbed solar radiation would be.
The effect would be greatest during low wind conditions, when vertical mixing of the water is weakest.
From what little I know of ocean modelling, this potential effect which I presume is what people are talking about is not taken into account. I suspect it would have to be parameterized, because it occurs on such a small scale. As far as I know, a Watt of IR is treated the same as a Watt of solar in the energy budget of the top ocean layer in a model.
I would like to hear what others know about this issue. I suspect it is something that would have to be investigated with a controlled experiment of some sort.
End quote
This is a simple yet incredibly important part of climate physics. Yet it is not 'settled science' or even modeled in the GCMs. The one point I am especially intrigued by is whether one watt of solar is equivalent to one watt IR.
I frequently see the assertion made that infrared (IR) radiation cannot warm a water body because IR only affects the skin (microns of water depth), whereas solar radiation is absorbed over water depths of meters to tens of meters.
Before discussing the issue, though, we first must agree that temperature and temperature change of a body is related to rates of energy gain and energy loss by that body. If we cannot agree on the basic concept that temperature changes when energy gain does not equal energy loss, then there is no basis for further discussion.
If the surface of a water body is emitting IR, then IR must be part of its energy budget, and therefore of its temperature. Evaporation only occurs at the skin, and we know that evaporation is the major component of heat loss by water bodies. How is it that evaporation can perform this function, and IR cannot?
The temperature of land clearly is affected by IR, and that only occurs at the surface of the soil. So, how can IR affect land temperature and not ocean temperature?
If you claim that any additional IR (say, due to increasing carbon dioxide) is immediately lost by the water body through evaporation, how exactly does that occur? The surface doesnt know why it has the temperature it does, it will evaporate water based (partly) on surface temperature, and it does not distinguish where the heat comes from (solar radiation from above, mixing from below, IR from above, sensible heat flux across the air/water interface). To claim that any energy gain from IR is immediately lost by evaporation is just an assertion.
NEVERTHELESS
It might well be that solar radiation is more efficient (on a Watt per Watt basis) than IR radiation at changing ocean temperature. In other words, that IR warming of a water body is more likely to be lost through evaporation, since its warming effect does occur only at the surface, and so that energy is more likely to be lost through evaporation than absorbed solar radiation would be.
The effect would be greatest during low wind conditions, when vertical mixing of the water is weakest.
From what little I know of ocean modelling, this potential effect which I presume is what people are talking about is not taken into account. I suspect it would have to be parameterized, because it occurs on such a small scale. As far as I know, a Watt of IR is treated the same as a Watt of solar in the energy budget of the top ocean layer in a model.
I would like to hear what others know about this issue. I suspect it is something that would have to be investigated with a controlled experiment of some sort.
End quote
This is a simple yet incredibly important part of climate physics. Yet it is not 'settled science' or even modeled in the GCMs. The one point I am especially intrigued by is whether one watt of solar is equivalent to one watt IR.