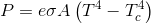- Thread starter
- #181
hahahaha. Ok, start putting up the actual observations. I think you will find reality is not so cut and dried as you present it.
Geez ian, how dense are you...try actually observing that if you want to ACTUALLY measure radiation in the bands of the so called greenhouse gasses moving from the atmosphere to the surface, the instrument must be cooled to something like -80F...then you aren't measuring IR in those bands moving from the cool atmosphere to the warmer surface of the earth..you are measuring IR moving from the warmer atmosphere to the cooler instrument...set an instrument at ambient temperature right next to it and it won't be measuring any such radiation...
why are you not putting up evidence?
you keep saying every observation, etc,etc,etc supports your position. start making your case. the time is long past that we are going to take your word for anything. as well, just about every link that you have presented so far either disagrees with your premise, is completely off topic, or is a blog opinion piece.
first off, show us half a dozen instruments that need to be cooled to -80F. link up to the documentation that says the cooling is necessary for the method to work rather than just an improvement to lower the time to make a reading or to increase the sensitivity by blocking out background noise from waste heat contamination.
you keep bringing up -80F. what is the method to reach this temperature? what is the cutoff of the IR bands that would require even more cooling?
it should be easy for you to post pictures of these instruments, and the working environment. please cut and paste relevant sections of the documentation instead of just dropping a link.
it's not that hard. I know I did it to support my position.