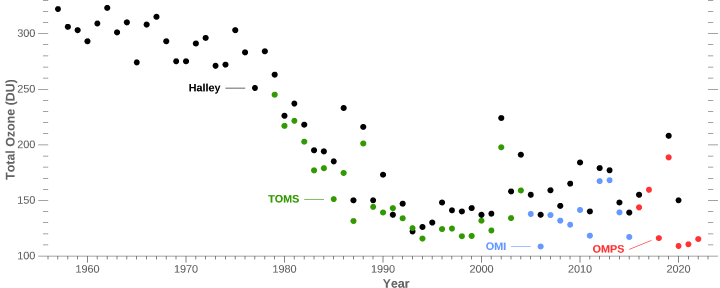
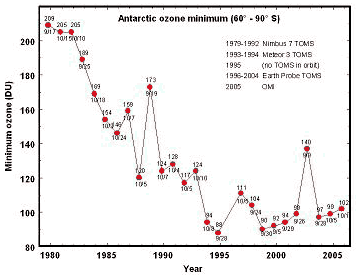
Notice that warmists ignore the fact that the rest of the worlds ozone cover outside of the polar region hardly changed at all the whole time. I doubt they even know about the EXISTENCE of monitoring stations that measures Ozone on a daily basis.
Why did you think it should change significantly? That's not what the science says should happen, after all.
As is always the case, you failed at the basics. But then, your conspiracy cult commands you to get everything wrong, and you serve the cult admirably.