Stryder50
Platinum Member
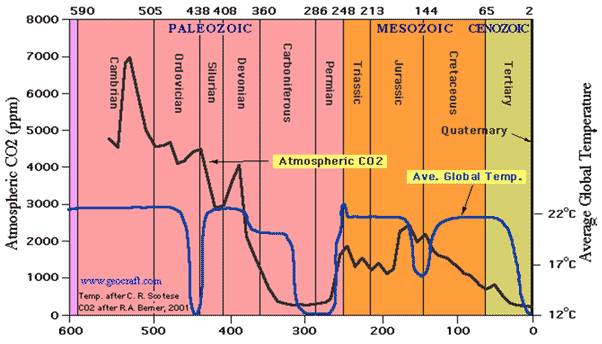
Except I didn't say anything about the source of CO2 being at issue, rather the disinformation that CO2 itself is significant factor. It's not even a minor factor.I'm afraid it is volcanism's CO2 output that is negligible.
Which emits more carbon dioxide: volcanoes or human activities?
Human activities emit 60 or more times the amount of carbon dioxide released by volcanoes each year.www.climate.gov
Human activities emit 60 or more times the amount of carbon dioxide released by volcanoes each year. Large, violent eruptions may match the rate of human emissions for the few hours that they last, but they are too rare and fleeting to rival humanity’s annual emissions. In fact, several individual U.S. states emit more carbon dioxide in a year than all the volcanoes on the planet combined do.
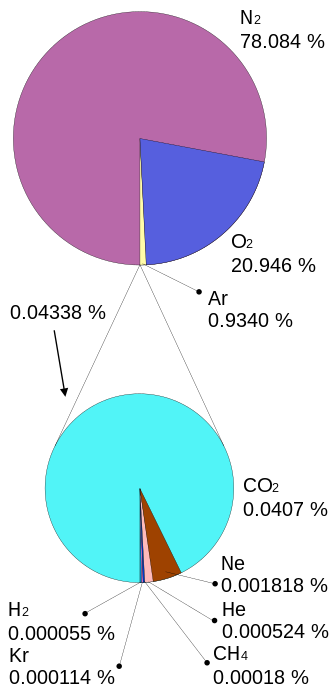
Unless you can show-demonstrate (such as via laboratory experiments) how one molecule three degrees warm than adjacent 2,499 molecules can transfer any heat beyond 0.000000x degrees to them, CO2 as a cause of Global Warming, or Atmosphere Warming does not happen!