munkle
Diamond Member
- Dec 18, 2012
- 5,061
- 8,578
- 2,130
So when do the apologies come for all those elderly people who died but didn't have to.

 childrenshealthdefense.org
childrenshealthdefense.org
“The U.S. Food and Drug Administration (FDA) agreed to take down its website and social media posts warning people not to use ivermectin to treat COVID-19 under terms of a settlement reached Thursday in a lawsuit alleging the agency exceeded its authority when it directed health professionals and patients not to use the drug.
Within 21 days, the agency will remove the consumer update, “Why You Should Not Use Ivermectin to Treat or Prevent COVID-19,” which pictures a doctor and a horse. The FDA posted the update on March 5, 2021.
The FDA webpage, still live, states repeatedly that the FDA has not authorized or approved ivermectin for treating COVID-19 and warns the drug can be “unsafe.” The page also includes language warning people not to use ivermectin “intended for livestock.”
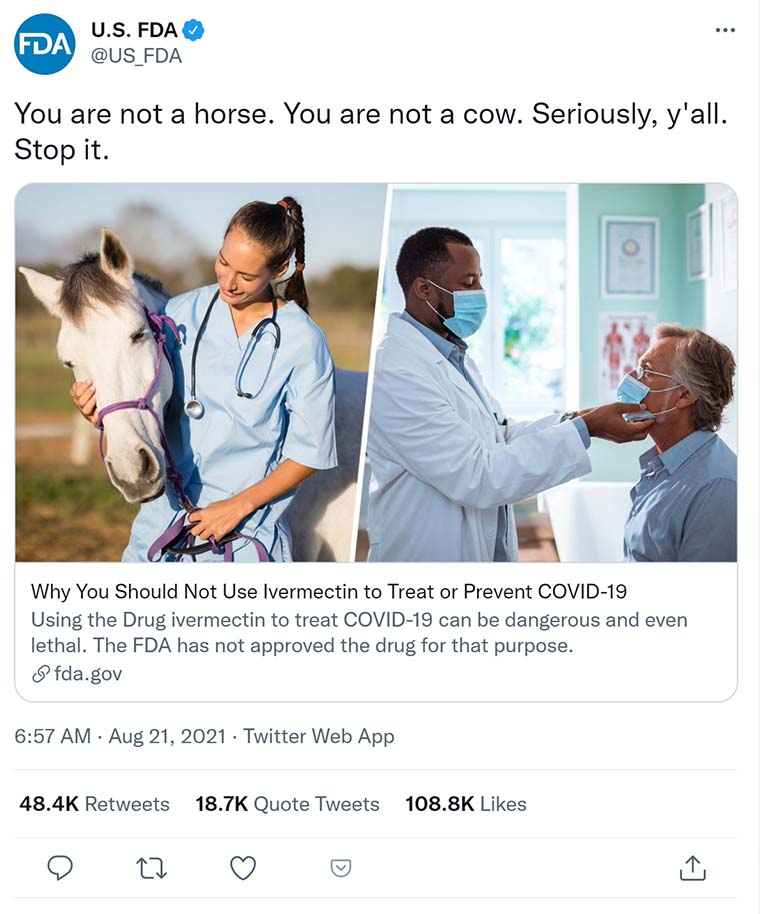
The FDA will also delete social media posts from Twitter, LinkedIn, Facebook and Instagram posted in 2021 and 2022 with messages such as “You are not a horse. You are not a cow. Seriously, y’all. Stop it.””
Below: FDA post on Twitter implying that Ivermetin was only for livestock
A compendium of existing scientific literature on the use of Ivermectin for COVID, c19ivm.org, has long reported since the start of COVID that when Ivermectin is used:
“Statistically significant lower risk is seen for mortality, ventilation, ICU admission, hospitalization, recovery, cases, and viral clearance. All remain significant for higher quality studies. 61 studies from 55 independent teams in 25 different countries show statistically significant improvements.
Meta analysis using the most serious outcome shows 62% [51‑70%] and 85% [77‑90%] lower risk for early treatment and prophylaxis, with similar results for higher quality studies, primary outcomes, peer-reviewed studies, and for RCTs.
Results are very robust — in worst case exclusion sensitivity analysis 61 of 101 studies must be excluded to avoid finding statistically significant efficacy.”

‘Blood on Its Hands’: FDA Will Remove Anti-ivermectin Social Media, Website Posts Under Lawsuit Settlement Agreement
The FDA, which denied any wrongdoing, said it will remove content warning people not to use ivermectin to treat COVID-19 within 21 days. Plaintiffs called the settlement a big win for patients and the patient-physician relationship.
“The U.S. Food and Drug Administration (FDA) agreed to take down its website and social media posts warning people not to use ivermectin to treat COVID-19 under terms of a settlement reached Thursday in a lawsuit alleging the agency exceeded its authority when it directed health professionals and patients not to use the drug.
Within 21 days, the agency will remove the consumer update, “Why You Should Not Use Ivermectin to Treat or Prevent COVID-19,” which pictures a doctor and a horse. The FDA posted the update on March 5, 2021.
The FDA webpage, still live, states repeatedly that the FDA has not authorized or approved ivermectin for treating COVID-19 and warns the drug can be “unsafe.” The page also includes language warning people not to use ivermectin “intended for livestock.”
The FDA will also delete social media posts from Twitter, LinkedIn, Facebook and Instagram posted in 2021 and 2022 with messages such as “You are not a horse. You are not a cow. Seriously, y’all. Stop it.””
Below: FDA post on Twitter implying that Ivermetin was only for livestock

A compendium of existing scientific literature on the use of Ivermectin for COVID, c19ivm.org, has long reported since the start of COVID that when Ivermectin is used:
“Statistically significant lower risk is seen for mortality, ventilation, ICU admission, hospitalization, recovery, cases, and viral clearance. All remain significant for higher quality studies. 61 studies from 55 independent teams in 25 different countries show statistically significant improvements.
Meta analysis using the most serious outcome shows 62% [51‑70%] and 85% [77‑90%] lower risk for early treatment and prophylaxis, with similar results for higher quality studies, primary outcomes, peer-reviewed studies, and for RCTs.
Results are very robust — in worst case exclusion sensitivity analysis 61 of 101 studies must be excluded to avoid finding statistically significant efficacy.”