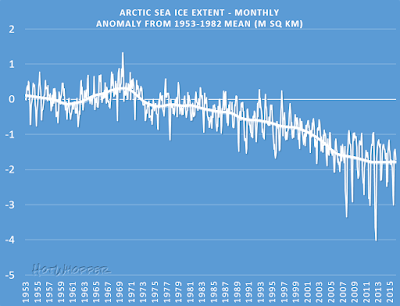
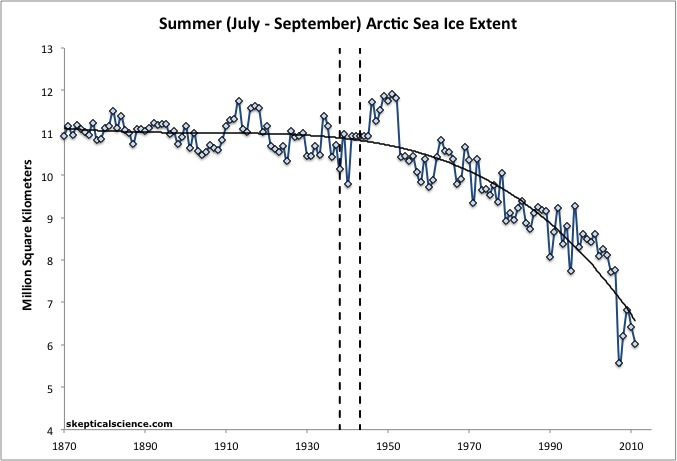
- May 20, 2009
- 144,521
- 66,933
- 2,330
Same Shit Different Day (SSDD) has been denying the mountains of empirical and theoretical evidence since day 1. When pointed out to the nearly two thousand pages of it to be found in "The Physical Science Basis" by Working Group I of the IPCC, he will first try to tell us there is nothing there because we choose not to spoon feed it to him, then will claim it is not empirical and is all based on flawed models, then will claim all the temperature data is falsified.
He is useless.
What you have proven crick...is that you are unable to differentiate between evidence showing that the climate changes...and evidence showing a cause for the change...you provide evidence of a changing climate and assume that man must be the cause...I have asked for observed, measured, quantified, empirical evidence supporting the AGW hypothesis over natural variability...and neither you, nor any of your fellow glassy eyed cultist buds here...nor all of climate science can provide the first bit...
And your response is to A) lie your ass off because you know there is no such evidence in existence or B) prove beyond any doubt how f'ing stupid you are by providing post after post of material that may prove change, but doesn't even take the first step in proving cause.
I lean towards you being a f'ing stupid liar.
The climate changes and they claim they've eliminated all variables except for a teeny, tiny atmospheric trace element. But ask for one lab experiment and you get the most bizarre and insane reasons why they can't work in a lab