I have posted on other threads Britannica's list of carbon in sediments, rocks, etc - e.g. over 64 million petagrams of carbon in earth's crustal carbonate deposits (primarily Calcium carbonate = Calcite = CaCO3 in Limestone deposited by the geologic carbon cycle which requires a massive carbon dioxide atmosphere being dissolved by earth's primordial oceans and then combining with Calcium ions in the ocean to produce CaCO3 [along with other carbonates]). Chemical evolutionists ignore those Calcium ions in earth's primordial ocean because those ions would destroy molecules on potential chemical pathways to amino acids (in proteins) and nucleic acids (in RNA).
However, there are many other minerals in earth's crust, as well as sediments (e.g. sandstone - sand is mostly Silicon Dioxide [SiO2] which is oxidized Silicon).
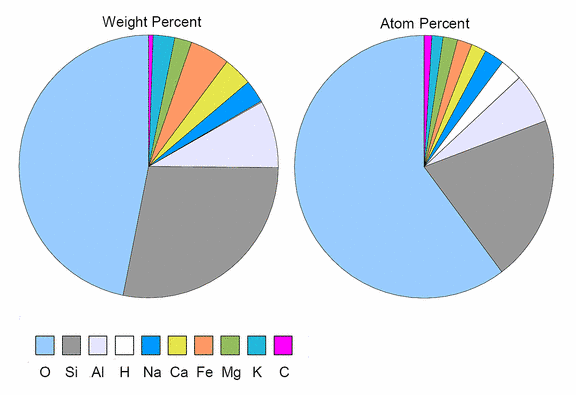
Oxygen is the most abundant element in earth's crust, Silicon is not far behind.
In my next post I will begin listing common (and some very beautiful) minerals in earth's crust - feel free to post anything relevant to thread title you all!
However, there are many other minerals in earth's crust, as well as sediments (e.g. sandstone - sand is mostly Silicon Dioxide [SiO2] which is oxidized Silicon).
Oxygen is the most abundant element in earth's crust, Silicon is not far behind.
In my next post I will begin listing common (and some very beautiful) minerals in earth's crust - feel free to post anything relevant to thread title you all!