daveman
Diamond Member
Apparently, 4 degrees spells climate doom | Watts Up With That?
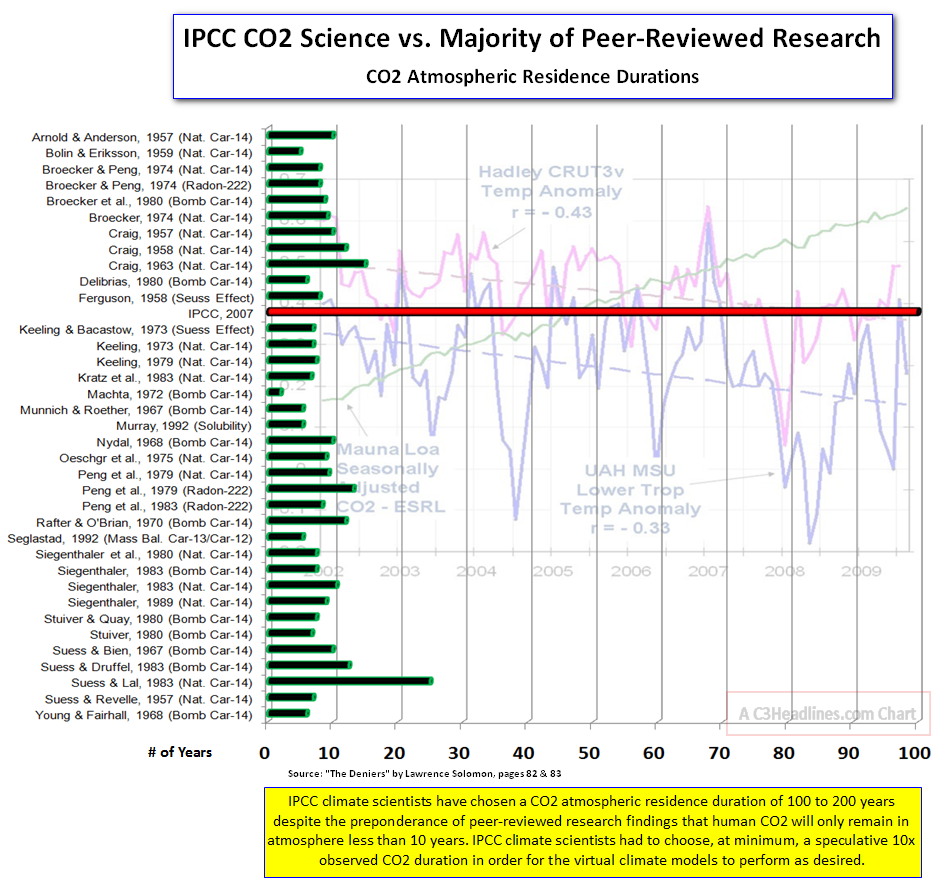
That's what happens when you try to make reality fit your model, instead of making your model fit reality -- as is done in normal science.

That's what happens when you try to make reality fit your model, instead of making your model fit reality -- as is done in normal science.



