Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.

Note: This feature currently requires accessing the site using the built-in Safari browser.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
What the science says
- Thread starter Crick
- Start date
- May 20, 2009
- 144,048
- 66,318
- 2,330
That much
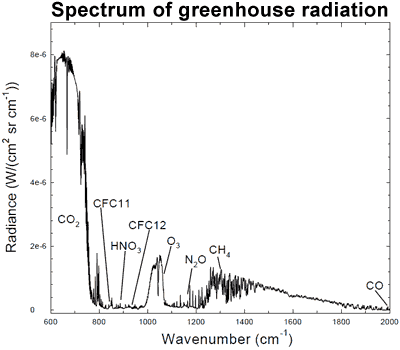
And I'd like an explanation of what you think your "emission peak" statement actually means.
LOL... wheres the temperature axis?
'It is embedded in your last post...
Are you recinding that statement?
No.
I could just as easily infer that your theory supposes that the non-excited gas will pop off a photon to its excited neighbors
Well, yes, that could happen.
The molecules are constantly colliding with each other. We know the average speed from the temperature but we don't know the speed of any individual molecule precisely unless we measure it, which of course would change the speed.
A molecule that absorbs a photon gains POTENTIAL energy (plus the small amount of momentum that is a fundamental of entropy). During the next molecular collision the excited molecule may or may not give up the potential energy, either to another molecule's potential energy; or more likely ,add that energy to the pool of kinetic energy. Adding to kinetic energy is by definition warming the temperature. AKA thermalization of radiation.
The opposite also happens. Closer to the top of the atmosphere, collisions excite the CO2 molecules but because there are fewer collisions it is more likely that the molecules will stay excited long enough to re-emit and that the reemission will escape to space.
Therefore CO2 tends to warm the atmosphere lower down but cool the atmosphere higher up.
Increasing CO2 concentration decreases the height to extinction of certain IR bands radiated by the surface. And raises the height in the upper atmosphere where radiation can escape, which is typically cooler and therefore less radiation.
I am not saying I believe the IPCC consensus position. I am saying that I believe CO2 has a warming influence.
CO2 doesn't warm the atmosphere at all. You believe in the magic..you just believe it isn't as strong as the off the deep end wackos believe...but belief in magic is belief in magic...even if it is belief in weak magic.
CO2 doesn't warm the atmosphere at all.
Does it absorb IR from the surface? Does it collide with other molecules?
About 1 in a billion CO2 molecules absorbs a bit of IR radiation from the surface and then emits it...along a temperature gradient which is always moving from warm to cool...the other nine hundred ninety nine million nine hundred ninety nine thousand nine hundred and ninety nine CO2 molecules pick up a bit of energy via collisions just like O2 and N2 and pass it along via conduction. CO2 acts as a hole in the blanket allowing some small bit of energy to move on out of the atmosphere at a much quicker rate than does convection and conduction.
And what warms the atmosphere is energy...not CO2.
absorbs a bit of IR radiation from the surface and then emits it...along a temperature gradient which is always moving from warm to cool...
Why do you feel that?
SSDD
Gold Member
- Nov 6, 2012
- 16,672
- 1,965
- 280
That much

And I'd like an explanation of what you think your "emission peak" statement actually means.
Where is the temperature axis of your supposed proof?...
Radiating Temperature
(Tr), a physical parameter characterizing the total (for all wavelengths) radiant emittance Be of a radiating body. It is equal to the temperature of a black-body at which the blackbody’s emittance

The laws of thermal radiation permit the expression

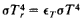
So this tells us that the peak radiating temperature of CO2 is -80C....now again....how much warming do you think that causes?
SSDD has as much problems collecting inferred knowledge from graphs as Crick does.
Satellites measuring outgoing radiation from the earth 'see' CO2 specific radiation coming from a source that appears to be -80C. So what does that mean?
It means that CO2 specific radiation cannot pass through the atmosphere and escape to space until it reaches a height in the atmosphere where the density of the air is so thin that it is no longer likely to absorb that radiation. How high? The layer that corresponds to -80C. (Yes I know it is a fuzzy boundary)
Until that point any radiation emitted by CO2 is retained by the atmosphere, the Greenhouse Effect.
The so-called Atmospheric Window allows radiation around 10 microns to escape directly to space. This radiation is not part of the Greenhouse Effect. Where do the satellites 'see' this radiation coming from? The layer coming from a temperature of +15C, the surface.
Satellites measuring outgoing radiation from the earth 'see' CO2 specific radiation coming from a source that appears to be -80C. So what does that mean?
It means that CO2 specific radiation cannot pass through the atmosphere and escape to space until it reaches a height in the atmosphere where the density of the air is so thin that it is no longer likely to absorb that radiation. How high? The layer that corresponds to -80C. (Yes I know it is a fuzzy boundary)
Until that point any radiation emitted by CO2 is retained by the atmosphere, the Greenhouse Effect.
The so-called Atmospheric Window allows radiation around 10 microns to escape directly to space. This radiation is not part of the Greenhouse Effect. Where do the satellites 'see' this radiation coming from? The layer coming from a temperature of +15C, the surface.
- Dec 18, 2013
- 136,155
- 27,798
- 2,180
no such thing as a greenhouse gas. Sorry Ian. you can't prove it. I laugh as I see you and others in here now agreeing that the molecules collide. You even agree with it, that means they don't emit when they collide correct?SSDD has as much problems collecting inferred knowledge from graphs as Crick does.
Satellites measuring outgoing radiation from the earth 'see' CO2 specific radiation coming from a source that appears to be -80C. So what does that mean?
It means that CO2 specific radiation cannot pass through the atmosphere and escape to space until it reaches a height in the atmosphere where the density of the air is so thin that it is no longer likely to absorb that radiation. How high? The layer that corresponds to -80C. (Yes I know it is a fuzzy boundary)
Until that point any radiation emitted by CO2 is retained by the atmosphere, the Greenhouse Effect.
The so-called Atmospheric Window allows radiation around 10 microns to escape directly to space. This radiation is not part of the Greenhouse Effect. Where do the satellites 'see' this radiation coming from? The layer coming from a temperature of +15C, the surface.
no such thing as a greenhouse gas. Sorry Ian. you can't prove it. I laugh as I see you and others in here now agreeing that the molecules collide. You even agree with it, that means they don't emit when they collide correct?SSDD has as much problems collecting inferred knowledge from graphs as Crick does.
Satellites measuring outgoing radiation from the earth 'see' CO2 specific radiation coming from a source that appears to be -80C. So what does that mean?
It means that CO2 specific radiation cannot pass through the atmosphere and escape to space until it reaches a height in the atmosphere where the density of the air is so thin that it is no longer likely to absorb that radiation. How high? The layer that corresponds to -80C. (Yes I know it is a fuzzy boundary)
Until that point any radiation emitted by CO2 is retained by the atmosphere, the Greenhouse Effect.
The so-called Atmospheric Window allows radiation around 10 microns to escape directly to space. This radiation is not part of the Greenhouse Effect. Where do the satellites 'see' this radiation coming from? The layer coming from a temperature of +15C, the surface.
God but you're an idiot.
I couldnt be bothered to explain to you again. Co2 molecules that absorb a photon are likely to pass that energy to the energy pool of the atmosphere via collision. CO2 molecules that do emit a photon are likely to have received the energy for the photon from the energy pool of the atmosphere via collisions
- Dec 18, 2013
- 136,155
- 27,798
- 2,180
hence no back radiation. thanks!!no such thing as a greenhouse gas. Sorry Ian. you can't prove it. I laugh as I see you and others in here now agreeing that the molecules collide. You even agree with it, that means they don't emit when they collide correct?SSDD has as much problems collecting inferred knowledge from graphs as Crick does.
Satellites measuring outgoing radiation from the earth 'see' CO2 specific radiation coming from a source that appears to be -80C. So what does that mean?
It means that CO2 specific radiation cannot pass through the atmosphere and escape to space until it reaches a height in the atmosphere where the density of the air is so thin that it is no longer likely to absorb that radiation. How high? The layer that corresponds to -80C. (Yes I know it is a fuzzy boundary)
Until that point any radiation emitted by CO2 is retained by the atmosphere, the Greenhouse Effect.
The so-called Atmospheric Window allows radiation around 10 microns to escape directly to space. This radiation is not part of the Greenhouse Effect. Where do the satellites 'see' this radiation coming from? The layer coming from a temperature of +15C, the surface.
God but you're an idiot.
I couldnt be bothered to explain to you again. Co2 molecules that absorb a photon are likely to pass that energy to the energy pool of the atmosphere via collision. CO2 molecules that do emit a photon are likely to have received the energy for the photon from the energy pool of the atmosphere via collisions
- Dec 18, 2013
- 136,155
- 27,798
- 2,180
well if molecules are colliding, they aren't emitting. I thought you said it properly. shit even thanked yo.Yet another fact free, illogical declarative statement from jc. Care to explain the missing section before 'hence'?
Of course not.
well if molecules are colliding, they aren't emitting. I thought you said it properly. shit even thanked yo.Yet another fact free, illogical declarative statement from jc. Care to explain the missing section before 'hence'?
Of course not.
well if molecules are colliding, they aren't emitting.
If they aren't emitting, that IR isn't escaping to space.
What did I say earlier about slowing loss of heat? LOL!
- Dec 18, 2013
- 136,155
- 27,798
- 2,180
well it ain't because of back radiation if it isn't emitting.well if molecules are colliding, they aren't emitting. I thought you said it properly. shit even thanked yo.Yet another fact free, illogical declarative statement from jc. Care to explain the missing section before 'hence'?
Of course not.
well if molecules are colliding, they aren't emitting.
If they aren't emitting, that IR isn't escaping to space.
What did I say earlier about slowing loss of heat? LOL!
- May 20, 2009
- 144,048
- 66,318
- 2,330
SSDD has as much problems collecting inferred knowledge from graphs as Crick does.
Satellites measuring outgoing radiation from the earth 'see' CO2 specific radiation coming from a source that appears to be -80C. So what does that mean?
It means that CO2 specific radiation cannot pass through the atmosphere and escape to space until it reaches a height in the atmosphere where the density of the air is so thin that it is no longer likely to absorb that radiation. How high? The layer that corresponds to -80C. (Yes I know it is a fuzzy boundary)
Until that point any radiation emitted by CO2 is retained by the atmosphere, the Greenhouse Effect.
The so-called Atmospheric Window allows radiation around 10 microns to escape directly to space. This radiation is not part of the Greenhouse Effect. Where do the satellites 'see' this radiation coming from? The layer coming from a temperature of +15C, the surface.
And "retained by the atmosphere" you mean that the excited CO2 is hotter than it's non-excited neighbor?
well it ain't because of back radiation if it isn't emitting.well if molecules are colliding, they aren't emitting. I thought you said it properly. shit even thanked yo.Yet another fact free, illogical declarative statement from jc. Care to explain the missing section before 'hence'?
Of course not.
well if molecules are colliding, they aren't emitting.
If they aren't emitting, that IR isn't escaping to space.
What did I say earlier about slowing loss of heat? LOL!
You don't think collisions only reduce the energy in CO2, do you?
- Dec 18, 2013
- 136,155
- 27,798
- 2,180
why?well it ain't because of back radiation if it isn't emitting.well if molecules are colliding, they aren't emitting. I thought you said it properly. shit even thanked yo.Yet another fact free, illogical declarative statement from jc. Care to explain the missing section before 'hence'?
Of course not.
well if molecules are colliding, they aren't emitting.
If they aren't emitting, that IR isn't escaping to space.
What did I say earlier about slowing loss of heat? LOL!
You don't think collisions only reduce the energy in CO2, do you?
SSDD
Gold Member
- Nov 6, 2012
- 16,672
- 1,965
- 280
SSDD has as much problems collecting inferred knowledge from graphs as Crick does.
Satellites measuring outgoing radiation from the earth 'see' CO2 specific radiation coming from a source that appears to be -80C. So what does that mean?
It means that CO2 specific radiation cannot pass through the atmosphere and escape to space until it reaches a height in the atmosphere where the density of the air is so thin that it is no longer likely to absorb that radiation. How high? The layer that corresponds to -80C. (Yes I know it is a fuzzy boundary)
Until that point any radiation emitted by CO2 is retained by the atmosphere, the Greenhouse Effect.
The so-called Atmospheric Window allows radiation around 10 microns to escape directly to space. This radiation is not part of the Greenhouse Effect. Where do the satellites 'see' this radiation coming from? The layer coming from a temperature of +15C, the surface.
No ian..it just moves on via conduction...radiation is such a small part of moving IR to the upper atmosphere that it is nearly irrelevant.
SSDD
Gold Member
- Nov 6, 2012
- 16,672
- 1,965
- 280

a graph to go with my comment above. the white areas under the red line indicate how much radiation has been absorbed by the atmosphere. the large bite in the middle is from CO2
Not how much ian...just which frequencies...and again, the IR is absorbed and then emitted on towards a cooler area in the temperature gradient.
SSDD
Gold Member
- Nov 6, 2012
- 16,672
- 1,965
- 280
no such thing as a greenhouse gas. Sorry Ian. you can't prove it. I laugh as I see you and others in here now agreeing that the molecules collide. You even agree with it, that means they don't emit when they collide correct?SSDD has as much problems collecting inferred knowledge from graphs as Crick does.
Satellites measuring outgoing radiation from the earth 'see' CO2 specific radiation coming from a source that appears to be -80C. So what does that mean?
It means that CO2 specific radiation cannot pass through the atmosphere and escape to space until it reaches a height in the atmosphere where the density of the air is so thin that it is no longer likely to absorb that radiation. How high? The layer that corresponds to -80C. (Yes I know it is a fuzzy boundary)
Until that point any radiation emitted by CO2 is retained by the atmosphere, the Greenhouse Effect.
The so-called Atmospheric Window allows radiation around 10 microns to escape directly to space. This radiation is not part of the Greenhouse Effect. Where do the satellites 'see' this radiation coming from? The layer coming from a temperature of +15C, the surface.
God but you're an idiot.
I couldnt be bothered to explain to you again. Co2 molecules that absorb a photon are likely to pass that energy to the energy pool of the atmosphere via collision. CO2 molecules that do emit a photon are likely to have received the energy for the photon from the energy pool of the atmosphere via collisions
1 in a billion....and even then, the energy moves on to cooler pastures.
why?well it ain't because of back radiation if it isn't emitting.well if molecules are colliding, they aren't emitting. I thought you said it properly. shit even thanked yo.Yet another fact free, illogical declarative statement from jc. Care to explain the missing section before 'hence'?
Of course not.
well if molecules are colliding, they aren't emitting.
If they aren't emitting, that IR isn't escaping to space.
What did I say earlier about slowing loss of heat? LOL!
You don't think collisions only reduce the energy in CO2, do you?
Physics!
Similar threads
- Replies
- 95
- Views
- 1K
- Replies
- 27
- Views
- 467
- Replies
- 6
- Views
- 220
- Replies
- 261
- Views
- 3K
- Replies
- 25
- Views
- 396
Latest Discussions
- Replies
- 7K
- Views
- 47K
- Replies
- 1
- Views
- 3
Forum List
-
-
-
-
-
Political Satire 8013
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
ObamaCare 781
-
-
-
-
-
-
-
-
-
-
-
Member Usernotes 466
-
-
-
-
-
-
-
-
-
-