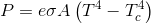Sunsettommy
Diamond Member
- Mar 19, 2018
- 14,894
- 12,528
- 2,400
Your question is framed in a nonsensical way. Net energy, heat, always flows from warm to cold.
So you keep saying...but you don't seem to be able to come up with an observed, measured example of it...what you come up with in spades is evidence that you are easily fooled by instrumentation.
And if all this were as simple as your "mind" experiments suggest, surely there would be observed, measured examples...we both know that there aren't...because your mind experiments are terribly flawed.
What a confusing statement this is:
"Net energy, heat, always flows from warm to cold."
How does it become net when energy flows in ONE direction from high to low, hot to cold and so on.
I am sorry you are so easily confused.
All heat is energy but energy is not always heat.
This conversation is mainly concerned with radiation. Radiation does not need matter to transport energy, only to produce it and to accept it. Unlike conduction and convection.
Therefore energy via radiation can, and does, travel in both (all) directions at the same time. Matter mediated energy transfer is only in one direction, at the level of the net competing energies.
SSDD thinks the temperature of the first object controls the production of radiation in the second object. And vice versa.
Bwahahahahahahahahahaha!!!
No Heat is NOT energy itself as shown here to help you out:
Heat shows up in boundary situations otherwise it is not called heat at all.
From Wikipedia is this easy to understand statement:
"n thermodynamics, heat is energy transferred from one system to another as a result of thermal interactions.[1] The amount of heat transferred in any process can be defined as the total amount of transferred energy excluding any macroscopic work that was done and any transfer of part of the object itself.[2][3][4][5][6] When two systems with different temperatures are put in contact, heat flows spontaneously from the hotter to the colder system. Transfer of energy as heat can occur through direct contact, through a barrier that is impermeable to matter (as in conduction), by radiation between separated bodies, by way of an intermediate fluid (as in convective circulation), or by a combination of these.[7][8][9] By contrast to work, heat involves the stochastic (random) motion of particles (such as atoms or molecules) that is equally distributed among all degrees of freedom, while work is confined to one or more specific degrees of freedom such as those of the center of mass."
bolding mine
Energy at rest shows NO heat at all, when it is being transferred it show up as heat, a simple concept you stumble over.