Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.

Note: This feature currently requires accessing the site using the built-in Safari browser.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
The EpiPen saga: Democrat CEO ripping off people in dire need...
- Thread starter MrMike
- Start date
MrMike
VIP Member
- May 12, 2015
- 242
- 63
- 80
- Thread starter
- #22
I'm noticing a pattern with piss drinkers like you. Trying to politicize this outrageous price hike and use it against Hillary? I smell desperation. Hey, that's the free market, pal. That's our system. The same system that allowed Trump to avoid paying federal taxes after losing almost a billion dollars.Anyone else notice any pattern$ when it comes to Libs/Clintons?
Ignoring your insult is easy. However, you fail to mention the fact the Clinton's have used the ClintonSlushFundation as a cash machine and have also used it to offset millions by writing off donations to it. In effect, they're using loopholes in the tax code. The other fact that this CEO and her company donated to their ClintonSlushFundation is just another interesting factoid.
You can go back to your special drink now...
Mylan recalls Epi-Pens within the U.S....

Device-maker expands recall of Mylan EpiPen, EpiPen Jr. to include U.S.
March 31, 2017 - Mylan's original recall on March 20 affected only devices shipped to locations outside the United States.

Device-maker expands recall of Mylan EpiPen, EpiPen Jr. to include U.S.
March 31, 2017 - Mylan's original recall on March 20 affected only devices shipped to locations outside the United States.
Meridian Medical Technologies, a manufacturer of Mylan's EpiPen auto-injectors, has expanded a recent voluntary recall to include some of the life-saving medical devices in the United States, officials said Friday. Mylan's original March 20 recall affected about 80,000 of the EpiPen devices shipped to locations outside the United States. Mylan NV announced that the additional recalled items were part of 13 lots of EpiPen (0.3 mg) and EpiPen Jr. (0.15 mg) 2-Pak Auto-Injectors that carry expiration dates between April and October. Mylan brands adult versions with a yellow label and junior devices with a green label.
The company said the recalls are the result of two reported EpiPen failures that occurred outside the United States -- due to a "potential defect in a supplier component" -- and that more than 80,000 auto-injectors could contain the fault. "The potential defect could make the device difficult to activate in an emergency and have significant health consequences for a patient experiencing a life-threatening allergic reaction," Mylan said in a statement Friday.
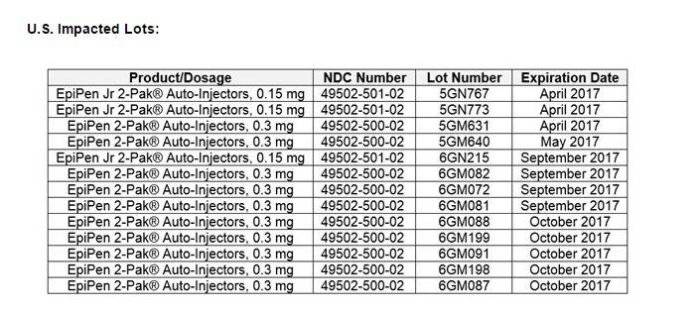
Mylan NV identified 13 lots of its EpiPen (0.3 mg) and EpiPen Jr. (0.15 mg) 2-packs shipped in the United States that are now included in a voluntary recall. The company said more than 80,000 of the life-saving auto-injectors are covered by the recall worldwide, which was prompted by two reports of device failures.
EpiPens administer doses of epinephrine to patients with anaphylaxis -- a serious allergic reaction that can be fatal if not treated immediately. Mylan said both reported failures included devices, manufactured between December 2015 and July 2016, that were already included in the original recall. No device failures have been reported in the United States. "The expanded voluntary recall is being initiated in the U.S. and also will extend to additional markets in Europe, Asia, North and South America," Mylan said.
The recalled items will be replaced at no cost. The company said the action is being done with the knowledge of the U.S. Food and Drug Administration. Customers should contact Mylan at (800) 796-9526 or email [email protected] if they have any questions.
Device-maker expands recall of Mylan EpiPen, EpiPen Jr. to include U.S.
New Generic EpiPen Wins FDA Approval...

New Generic EpiPen Wins FDA Approval
August 16, 2018 | WASHINGTON — U.S. health officials Thursday approved a new generic version of EpiPen, the emergency allergy medication that triggered a public backlash because of its rising price tag.

New Generic EpiPen Wins FDA Approval
August 16, 2018 | WASHINGTON — U.S. health officials Thursday approved a new generic version of EpiPen, the emergency allergy medication that triggered a public backlash because of its rising price tag.
The new version from Teva Pharmaceuticals is the first that will be interchangeable with the original penlike injector sold by Mylan. The Food and Drug Administration announced the approval in a statement. EpiPen injections are stocked by schools and parents nationwide to treat children with severe allergies. They are used in emergencies to stop potentially fatal allergic reactions to insect bites and stings and foods like nuts and eggs.
EpiPen maker Mylan has dominated the $1 billion market for the shots for two decades. Several other companies sell competing shots containing the drug epinephrine, but they aren’t heavily marketed or prescribed by doctors. In 2016, Congress blasted Mylan in letters and hearings for raising EpiPen’s to $600 for a two-pack, a five-fold increase over nearly a decade. The company responded by launching its own lower-cost generic version for $300.
A pharmacist holds a package of EpiPens epinephrine auto-injector, a Mylan product, in Sacramento, Calif., July 8, 2016. Mylan has a generic version of its emergency allergy treatment and a new competitor has been given FDA approval.
Mylan continues to sell both versions at those prices, according to data from Elsevier’s Gold Standard Drug Database. Teva’s generic shot will be the first version that pharmacists can substitute even when doctors prescribe the original EpiPen.
A Teva spokeswoman declined to comment on the drug’s price but said it would launch “in the coming months.” Generic drugs can be priced as much as 80 percent lower than the original product. But those price cuts usually appear after several companies have launched competing versions. Teva’s bid to sell a generic EpiPen faced multiple setbacks at the FDA, which rejected the company’s initial application in 2016. While epinephrine is a decades-old generic drug, Teva and other would-be competitors struggled to replicate the EpiPen’s auto-injector device.
New Generic EpiPen Wins FDA Approval
Biff_Poindexter
Diamond Member
Similar threads
- Replies
- 38
- Views
- 8K
- Replies
- 136
- Views
- 9K
- Replies
- 10
- Views
- 1K
- Replies
- 6
- Views
- 2K
Latest Discussions
- Replies
- 16K
- Views
- 276K
Forum List
-
-
-
-
-
Political Satire 8037
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
ObamaCare 781
-
-
-
-
-
-
-
-
-
-
-
Member Usernotes 468
-
-
-
-
-
-
-
-
-
-
