Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.

Note: This feature currently requires accessing the site using the built-in Safari browser.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Ocean Acidification Takes Another Hit
- Thread starter SSDD
- Start date
Delta4Embassy
Gold Member
Amusing all the climate change deniers posting their crap. Like if they do that that'll somehow change physical reality.
"Let's all post how it isn't happening on discussion sites. That way, people will eventually believe us. ...True things are still melting and hitting the fan, but people wont know about it." Or whatever crazy people think about.
"Let's all post how it isn't happening on discussion sites. That way, people will eventually believe us. ...True things are still melting and hitting the fan, but people wont know about it." Or whatever crazy people think about.
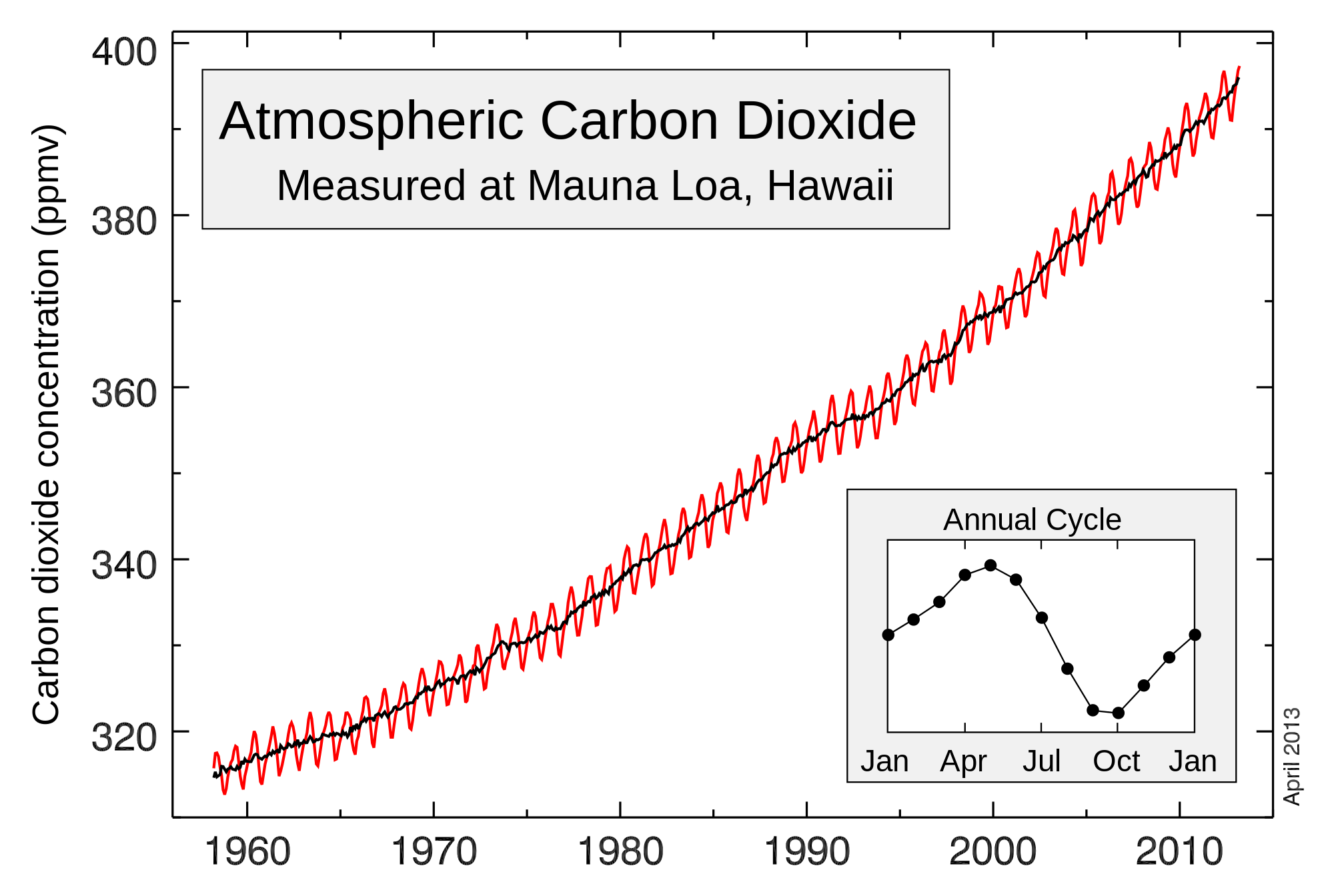
Still waiting though. Does ANYONE out there agree that 120 ppm CO2 is statistically insignficant or that it is below the resolution of instruments used to measure it? Does anyone else out there believe we can't actually detect 120 ppm change in CO2 level? Does anyone else out there think a 40% change in CO2 is "statistically insignificant"? Anyone?
SSDD
Gold Member
- Nov 6, 2012
- 16,672
- 1,965
- 280
- Thread starter
- #46
A 40% increase in a trace gas to which the climate is not sensitive is insignificant in so far as any discussion of the climate is concerned. .Still waiting though. Does ANYONE out there agree that 120 ppm CO2 is statistically insignficant or that it is below the resolution of instruments used to measure it? Does anyone else out there believe we can't actually detect 120 ppm change in CO2 level? Does anyone else out there think a 40% change in CO2 is "statistically insignificant"? Anyone?
- Dec 18, 2013
- 136,155
- 27,798
- 2,180
Well Jiminie, can you show it is significant? It is the open question that seems to escape your attention daily. K00ksville baby....WiNNiNg..What a fucking idiot.
Who here agrees with Billy Bob that 120 ppm is statistically insignificant and is less than the resolution of measurements?

- Dec 18, 2013
- 136,155
- 27,798
- 2,180
Thes k00ks are up at 4:00am in the morning posting on here and then wondering why no one has responded to their posts. I'm telling you these nutjobs are definitely clueless. This thread alone is that evidence. It's hilarious.A 40% increase in a trace gas to which the climate is not sensitive is insignificant in so far as any discussion of the climate is concerned. .Still waiting though. Does ANYONE out there agree that 120 ppm CO2 is statistically insignficant or that it is below the resolution of instruments used to measure it? Does anyone else out there believe we can't actually detect 120 ppm change in CO2 level? Does anyone else out there think a 40% change in CO2 is "statistically insignificant"? Anyone?

SwimExpert
Gold Member
- Nov 26, 2013
- 16,247
- 1,679
- 280
- Banned
- #49
SSDD, you're embarrassing yourself badly now, proudly displaying how hard you fail at such basic concepts like Henry's law. You'd literally flunk a freshman chemistry class. No wonder your cult found you to be such easy pickings.
Temperature. So you say, yet you still can't grasp: Temperature.
SwimExpert
Gold Member
- Nov 26, 2013
- 16,247
- 1,679
- 280
- Banned
- #50
What a fucking idiot.
Who here agrees with Billy Bob that 120 ppm is statistically insignificant and is less than the resolution of measurements?
Technically speaking, one cannot agree nor disagree without a proper frame of reference. If we were talking about 50,000 ppm, then 120 would be insignificant. If we were talking about 200 ppm then 120 would be very significant.
So your claiming pressure differences?
Before you embarrass yourself more, go read up on what "partial pressure" means. You're too 'effin stupid on this topic to be bothering the grownups.
Look, if you kooks had simply said "oops, we goofed", or even if you had just quietly crept away, that wouldn't be a big deal. Mistakes happen. It's what you do after the mistake that reveals your character.
In this case, after the deniers found they had made a mistake, they chose to lie about it and pretend no mistake had been made. And that's why I point out most deniers are pathologically dishonest. Their herd-loyalty always overrides their honesty. The herd here decided to lie about this, and none of the herd members has the guts to contradict the rest of the herd.
Old Rocks
Diamond Member
Actually, Mamooth, they have in the past lambasted the most egrerious of the people making errors. Like what they did to SSo DDumb and his one way photons.
- Dec 18, 2013
- 136,155
- 27,798
- 2,180
mistake, which mistake is that?So your claiming pressure differences?
Before you embarrass yourself more, go read up on what "partial pressure" means. You're too 'effin stupid on this topic to be bothering the grownups.
Look, if you kooks had simply said "oops, we goofed", or even if you had just quietly crept away, that wouldn't be a big deal. Mistakes happen. It's what you do after the mistake that reveals your character.
In this case, after the deniers found they had made a mistake, they chose to lie about it and pretend no mistake had been made. And that's why I point out most deniers are pathologically dishonest. Their herd-loyalty always overrides their honesty. The herd here decided to lie about this, and none of the herd members has the guts to contradict the rest of the herd.
A 40% increase in a trace gas to which the climate is not sensitive is insignificant in so far as any discussion of the climate is concerned.
It's not as if I expected you to suddenly gain basic intelligence. But listen up. All the gases in our atmosphere dissolve in the ocean. The amount that you'd find in solution when equilibrium is reached is dependent on two things: the temperature of the ocean and the partial pressure of the gas above the ocean. Increasing the ocean's temperature decreases solubility. Increasing partial pressure increases solubility. Both terms: temperature and partial pressure - are treated linearly in the van 't Hoff equation. The decreasing solubility caused by the minute increase in temperature that has taken place in the world's oceans in the last 150 years is outweighed by more than two orders of magnitude by the increase in CO2 partial pressure. And since CO2 reacts with water to form H2CO3, even larger amounts of gas than the idealized equations would indicate, can dissolve into water under any given circumstances.
The increasing levels of CO2 in our atmosphere are causing the world's oceans to grow more acidic. Arguing otherwise is simple stupidity.
SwimExpert
Gold Member
- Nov 26, 2013
- 16,247
- 1,679
- 280
- Banned
- #55
A 40% increase in a trace gas to which the climate is not sensitive is insignificant in so far as any discussion of the climate is concerned.
It's not as if I expected you to suddenly gain basic intelligence. But listen up. All the gases in our atmosphere dissolve in the ocean. The amount that you'd find in solution when equilibrium is reached is dependent on two things: the temperature of the ocean and the partial pressure of the gas above the ocean. Increasing the ocean's temperature decreases solubility. Increasing partial pressure increases solubility. Both terms: temperature and partial pressure - are treated linearly in the van 't Hoff equation. The decreasing solubility caused by the minute increase in temperature that has taken place in the world's oceans in the last 150 years is outweighed by more than two orders of magnitude by the increase in CO2 partial pressure. And since CO2 reacts with water to form H2CO3, even larger amounts of gas than the idealized equations would indicate, can dissolve into water under any given circumstances.
The increasing levels of CO2 in our atmosphere are causing the world's oceans to grow more acidic. Arguing otherwise is simple stupidity.
You started with point A. You've demonstrated point Z. But you still want us to take for granted B through Y.
- May 20, 2009
- 144,049
- 66,322
- 2,330
Still waiting though. Does ANYONE out there agree that 120 ppm CO2 is statistically insignficant or that it is below the resolution of instruments used to measure it? Does anyone else out there believe we can't actually detect 120 ppm change in CO2 level? Does anyone else out there think a 40% change in CO2 is "statistically insignificant"? Anyone?
Pick a fucking amount and show us in a lab how much of an increase it (doesn't) cause!
We're using 120PPM because the AGWCult insists that it causes a 2-7 degree temperature increase
Put up or shut the fuck up
Billy_Bob
Diamond Member
Still waiting though. Does ANYONE out there agree that 120 ppm CO2 is statistically insignficant or that it is below the resolution of instruments used to measure it? Does anyone else out there believe we can't actually detect 120 ppm change in CO2 level? Does anyone else out there think a 40% change in CO2 is "statistically insignificant"? Anyone?
Your a moron.. Please do the math. At 26 deg C, under 1000mb pressure, and concentration of 400ppm in the near surface, How much absorption of CO2 into the water will occur in a 24 hr period?
Average salinity of the water? What microbes are present? what other factors will increase or decrease absorption?
Crick, the amount absorbed is directly correlated to air movement, ambient air temp, water temp and waves. As this is different all over the world, the rates are different. You keep trying to make CO2 something its not. IN a static experiment as I indicated above there was no measurable dissolved CO2. At 26 Deg C CO2 uptake is immeasurable and if there was CO2 in the fluid, it would expand and rise to the surface. 120 ppm is insignificant except to plants that use it to grow.
Billy_Bob
Diamond Member
A 40% increase in a trace gas to which the climate is not sensitive is insignificant in so far as any discussion of the climate is concerned.
It's not as if I expected you to suddenly gain basic intelligence. But listen up. All the gases in our atmosphere dissolve in the ocean. The amount that you'd find in solution when equilibrium is reached is dependent on two things: the temperature of the ocean and the partial pressure of the gas above the ocean. Increasing the ocean's temperature decreases solubility. Increasing partial pressure increases solubility. Both terms: temperature and partial pressure - are treated linearly in the van 't Hoff equation. The decreasing solubility caused by the minute increase in temperature that has taken place in the world's oceans in the last 150 years is outweighed by more than two orders of magnitude by the increase in CO2 partial pressure. And since CO2 reacts with water to form H2CO3, even larger amounts of gas than the idealized equations would indicate, can dissolve into water under any given circumstances.
The increasing levels of CO2 in our atmosphere are causing the world's oceans to grow more acidic. Arguing otherwise is simple stupidity.
Please Tell me how they have returned to 1850 PH levels today? That rise you touted from just three station just off the left coast has now been shown incorrect. the mean pH of open ocean surface water is 7.9-8.3. The current rise you are fear-mongering is 0.01, well below statistical error bars.
IT is a localized event...
Source
Your a moron.. Please do the math. At 26 deg C, under 1000mb pressure, and concentration of 400ppm in the near surface, How much absorption of CO2 into the water will occur in a 24 hr period?
You first. You claim anyone who can't do the math is a moron, so do the math, otherwise you self-admit to being a moron. Be sure to show all your work.
IN a static experiment as I indicated above there was no measurable dissolved CO2.
Suuuuuure, Billy.
You're just making up stupid crap, demanding everyone believe it, and then crying when everyone laughs at you. If you want people to stop laughing at you, start backing up your bullshit.
Old Rocks
Diamond Member
Still waiting though. Does ANYONE out there agree that 120 ppm CO2 is statistically insignficant or that it is below the resolution of instruments used to measure it? Does anyone else out there believe we can't actually detect 120 ppm change in CO2 level? Does anyone else out there think a 40% change in CO2 is "statistically insignificant"? Anyone?
Your a moron.. Please do the math. At 26 deg C, under 1000mb pressure, and concentration of 400ppm in the near surface, How much absorption of CO2 into the water will occur in a 24 hr period?
Average salinity of the water? What microbes are present? what other factors will increase or decrease absorption?
Crick, the amount absorbed is directly correlated to air movement, ambient air temp, water temp and waves. As this is different all over the world, the rates are different. You keep trying to make CO2 something its not. IN a static experiment as I indicated above there was no measurable dissolved CO2. At 26 Deg C CO2 uptake is immeasurable and if there was CO2 in the fluid, it would expand and rise to the surface. 120 ppm is insignificant except to plants that use it to grow.
Link, Billy Boob, link. Otherwise, just more flap-yap.
Similar threads
- Replies
- 114
- Views
- 2K
- Replies
- 95
- Views
- 1K
- Replies
- 39
- Views
- 957
Latest Discussions
- Replies
- 42
- Views
- 199
- Replies
- 76
- Views
- 1K
- Replies
- 180
- Views
- 1K
Forum List
-
-
-
-
-
Political Satire 8013
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
ObamaCare 781
-
-
-
-
-
-
-
-
-
-
-
Member Usernotes 466
-
-
-
-
-
-
-
-
-
-