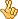New meds for Hep C...

New Drugs Promise Higher Cure Rates for Hepatitis C
June 10, 2011 - Medications recently approved for use in the US
New Drugs Promise Higher Cure Rates for Hepatitis C
June 10, 2011 - Medications recently approved for use in the US
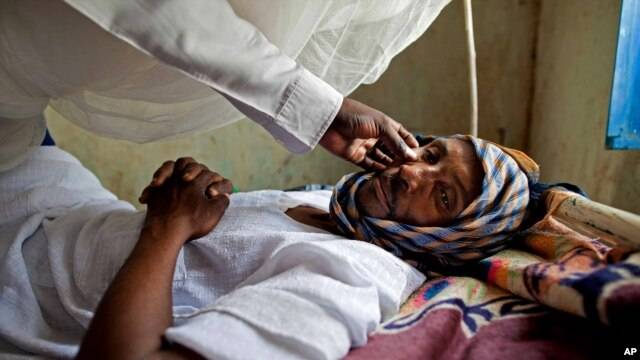
The U.S. Food and Drug Administration has approved two new drugs - boceprevir and telaprevir - to treat hepatitis C, a virus that can cause liver damage and cancer. The new drugs are expected to improve cure rates for some of the more than 170 million people worldwide affected by this potentially fatal disease. Hepatitis C is a blood-borne virus. If blood is not screened for diseases, the virus can be transmitted through transfusions or organ transplants. In the United States, the most common cause of infection is sharing drug-injection needles.
It can take years, or even decades, for the virus to cause enough liver damage to produce symptoms and about three-quarters of Americans with hepatitis C dont know they have it. People got their hepatitis C in the 60s and 70s, and early 80s, and now are having all these problems from it, 20 or 30 years later, says liver disease specialist Dr. Bruce Bacon of Saint Louis University, adding that about 20 percent of people exposed to hepatitis C recover from the virus on their own, without treatment. The flip side of that is about 80 percent of people - 80, 85 percent of people - do get chronic hepatitis. And of those patients, 20 to 30 percent of people develop cirrhosis, and of those, two to three percent of patients develop liver cancer.
There are six different strains, or genotypes, of hepatitis C. Saint Louis University researcher Dr. Adrian Di Bisceglie says that, in the U.S., about 70 percent of patients have genotype 1, a form of the virus that is also the most common in Latin America, Europe, and parts of Asia. And it turns out that genotype 1 is the most hard to treat, he says. For the past decade, treatment for hepatitis C has meant a year-long regimen of two drugs: an immune-system booster called pegylated interferon and an antiviral medication known as ribavirin.
But often, Di Besceglie says, that two-drug treatment hasnt worked for patients with the genotype 1 virus. In that genotype, only about 40 percent of patients get cured when theyre treated with interferon and ribavirin. In a clinical trial funded by the drug maker Vertex, Di Besceglie helped test how hard-to-treat genotype 1 patients responded when a new Vertex drug, telaprevir, was added to the current two-drug treatment. The cure rates or the rates of sustained virologic response go from the 40 percent number that I mentioned in patients with genotype 1, to 75 percent. A clinical trial for a similar medication, boceprevir, was funded by Merck, the drugs developer.
MORE