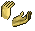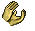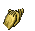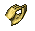Old Rocks
Diamond Member
What is Ocean Acidification?
The Biological Impacts
Ocean acidification is expected to impact ocean species to varying degrees. Photosynthetic algae and seagrasses may benefit from higher CO2 conditions in the ocean, as they require CO2 to live just like plants on land. On the other hand, studies have shown that a more acidic environment has a dramatic effect on some calcifying species, including oysters, clams, sea urchins, shallow water corals, deep sea corals, and calcareous plankton. When shelled organisms are at risk, the entire food web may also be at risk. Today, more than a billion people worldwide rely on food from the ocean as their primary source of protein. Many jobs and economies in the U.S. and around the world depend on the fish and shellfish in our oceans.
--------------------------------------------------------------------------------
Pteropods
The pteropod, or “sea butterfly”, is a tiny sea creature about the size of a small pea. Pteropods are eaten by organisms ranging in size from tiny krill to whales and are a major food source for North Pacific juvenile salmon. The photos below show what happens to a pteropod’s shell when placed in sea water with pH and carbonate levels projected for the year 2100. The shell slowly dissolves after 45 days. Photo credit: Used with permission, National Geographic Images.
The Biological Impacts
Ocean acidification is expected to impact ocean species to varying degrees. Photosynthetic algae and seagrasses may benefit from higher CO2 conditions in the ocean, as they require CO2 to live just like plants on land. On the other hand, studies have shown that a more acidic environment has a dramatic effect on some calcifying species, including oysters, clams, sea urchins, shallow water corals, deep sea corals, and calcareous plankton. When shelled organisms are at risk, the entire food web may also be at risk. Today, more than a billion people worldwide rely on food from the ocean as their primary source of protein. Many jobs and economies in the U.S. and around the world depend on the fish and shellfish in our oceans.
--------------------------------------------------------------------------------
Pteropods
The pteropod, or “sea butterfly”, is a tiny sea creature about the size of a small pea. Pteropods are eaten by organisms ranging in size from tiny krill to whales and are a major food source for North Pacific juvenile salmon. The photos below show what happens to a pteropod’s shell when placed in sea water with pH and carbonate levels projected for the year 2100. The shell slowly dissolves after 45 days. Photo credit: Used with permission, National Geographic Images.