Long wave IR, which as you know is what is returned to the surface from CO2 interference, is incapable of warming the oceans. That is KNOWN. So come up with a different mechanism to generate your heating.
Why the strawman?
You are a smart and educated man. Is it possible that you honestly don't understand my position after the hundreds of times that I have said that only the Sun heats the Earth surface? In dozens of ways?
Entropy (decay, increasing disorder) would be a lot easier for people to understand if we had a common word for the opposite (building up, increasing order). That is what makes life forms so amazing, they defy entropy at least for a short time.
Thermodynamics can be broken down into two types of processes. Passive, where every particle is trying to shed energy, every system is trying to shed heat in the most efficient way. And active, where an outside power source is adding energy and reversing entropy. There is of course a fuzzy boundary between the two. Is it new energy or just a redistribution of energy.
Ultimately, the Sun is our only power source (nuclear power on earth is just a remnant of a different star). Because the Earth is a spinning sphere it warms up roughly half the time and cools half the time. The surface is always passively trying to shed heat but it is only obvious to us when the Sun is not actively heating it. The surface is actually expelling more energy during the daylight heating phase, with a lag of course due to thermal inertia.
CO2 only effects the passive shedding of energy. It DOES NOT actively heat the surface, it reduces the surface passive cooling. There is no creation of extra energy, there is only a change in the distribution that leads to an accumulation energy in the atmosphere near the surface. This energy has just been 'borrowed' from space by not expelling it. Once equilibrium is again reached, the solar input matches terrestrial output again. The Earth's surface can have a wide range of average temperatures, and every one of them can be at equilibrium, it just depends on the conditions.
I think the total influence of CO2 from zero ppm to 400 ppm is probably about 8C. Extra CO2 has rapidly diminishing effect, but it still has an affect.
HOW does the CO2 have an effect? I agree that when dealing with an exoatmosphere, CO2 would have an effect, but in our atmosphere I have seen no evidence that it does.
Sorry, I don't get your point. Space doesn't care whether it goes without the temporarily retarded energy. It makes no difference.
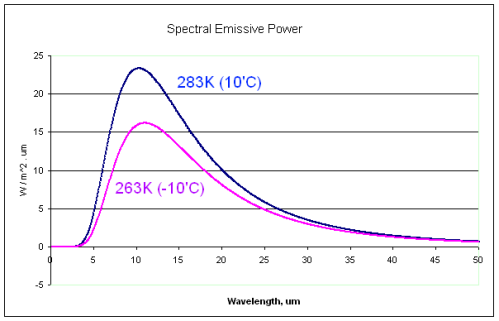
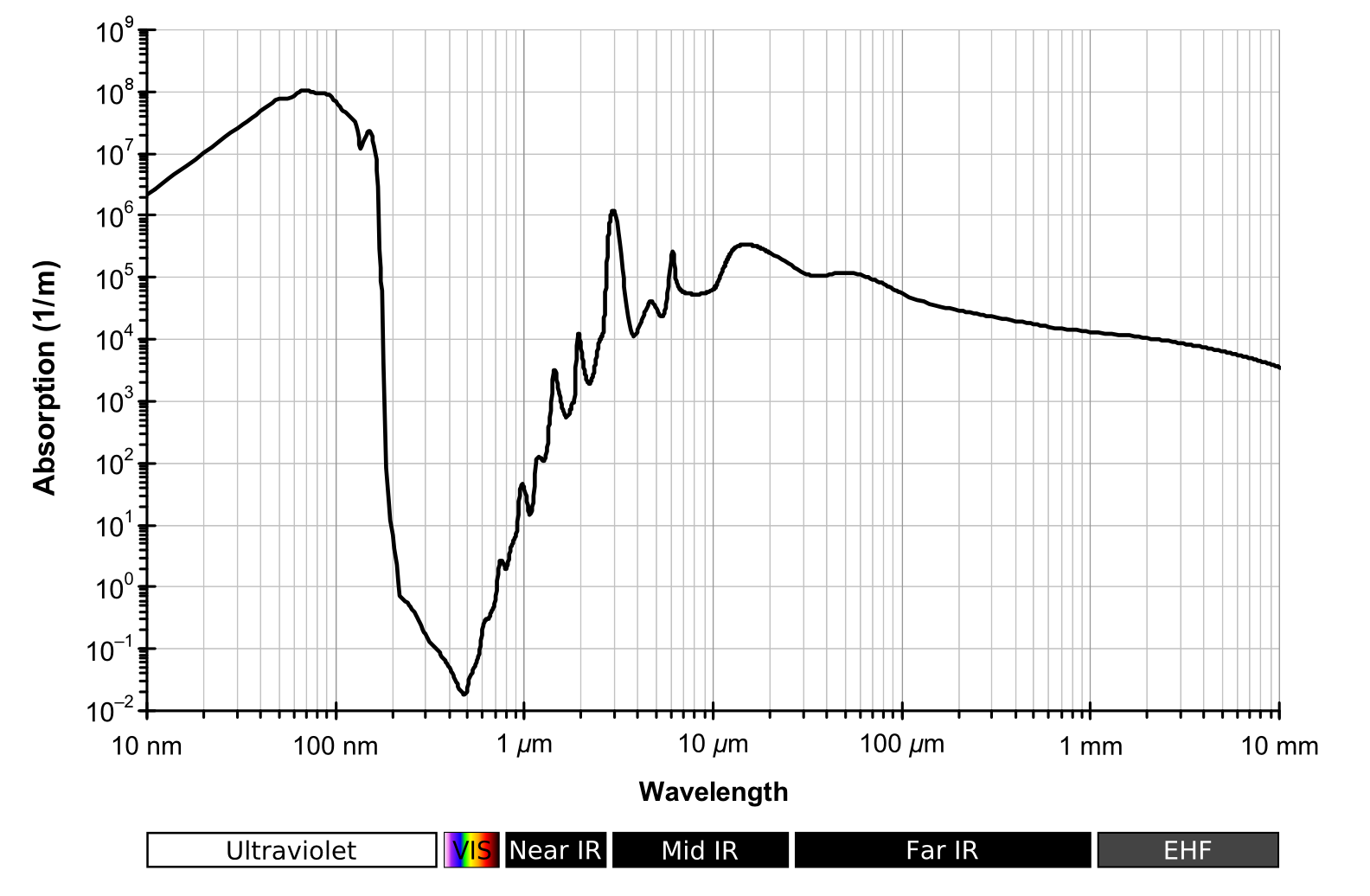
The mean free path for 15 micron radiation is two metres. We take surface temperature readings at 1.5 metres. Much of the surface radiated 15 micron has already been absorbed by that point. All of it by 10 metres. All of that surface energy has been immediately absorbed and converted to other forms of atmospheric energy. It then bounces around until much farther up where it finally starts escaping but from a cooler temperature. Absorption is not controlled by temperature but emission is. The difference between the amount that goes in at the bottom, and comes out at the top is the amount of energy that accumulates until it finds a new path out of the system.
Adding more CO2 shortens the mean free path. Which puts that energy into a smaller volume of air. And while the energy does find a different pathway out, there must be some change of temperature, or something, because the energy wasn't taking that path before.
My point is that in the complete absence of water vapor CO2 does indeed exert some power to warm. But in a dense water vapor atmosphere, such as ours, it doesn't. Whatever impact it could have was lost in the far more powerful water vapor signal. This belief that CO2 is the "control knob" is silly based on the observed scientific record.
You keep saying CO2 doesn't matter, at all.
I have no problem with admitting it is a small effect. I have said so myself on many occasions. I have also scoffed at the idea that CO2 is the control knob for climate.
Water is a much bigger player. With more mechanisms, and much great uncertainties. So what? How does that negate the effect of CO2?
Surface temperature is controlled by a myriad of factors and the interaction between them. Why do you think a simple and straight forward influence like CO2 should be removed? Shouldn't we be looking for more factors and interactions, rather than ignore one of the ones we know and understand to a fair degree?