Coral reefs around the world could be teetering on the brink of extinction by the end of the century as the oceans become more acidic, scientists have warned.
New evidence from volcanic seeps - fissures in the ocean floor that leak gases and minerals - suggests a bleak future for the reefs that harbour the world's richest marine ecosystems.
Three natural carbon dioxide (CO2) seeps in Papua New Guinea have given scientists a snapshot of how coral reefs may look in 100 years.
Like man-made sources of carbon dioxide, the seeps are making the water around them more acidic.
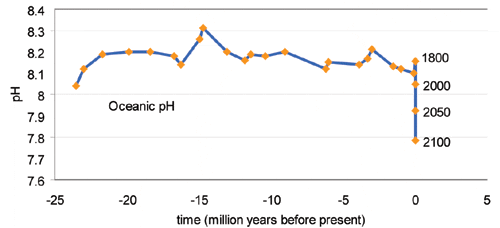
The study showed reductions in reef diversity and complexity as pH values fell from 8.1 to 7.8, indicating greater acidity. At values below 7.7, reef development ceased altogether.
Climate change experts estimate that by the end of the century, ocean acidity worldwide will change in a similar way because of CO2 emissions.
The latest Intergovernmental Panel on Climate Change (IPCC) forecast predicts that rising concentrations of CO2 will reduce worldwide ocean pH from its present level of 8.1 to 7.8.
Authors of the new research, writing in the journal Nature, said the effect of a pH drop below 7.8 would be "catastrophic" for the coral.
Coral reefs 'on edge of extinction' - Environment | MSN News - MSN UK
New evidence from volcanic seeps - fissures in the ocean floor that leak gases and minerals - suggests a bleak future for the reefs that harbour the world's richest marine ecosystems.
Three natural carbon dioxide (CO2) seeps in Papua New Guinea have given scientists a snapshot of how coral reefs may look in 100 years.
Like man-made sources of carbon dioxide, the seeps are making the water around them more acidic.
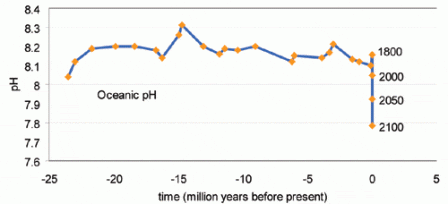
The study showed reductions in reef diversity and complexity as pH values fell from 8.1 to 7.8, indicating greater acidity. At values below 7.7, reef development ceased altogether.
Climate change experts estimate that by the end of the century, ocean acidity worldwide will change in a similar way because of CO2 emissions.
The latest Intergovernmental Panel on Climate Change (IPCC) forecast predicts that rising concentrations of CO2 will reduce worldwide ocean pH from its present level of 8.1 to 7.8.
Authors of the new research, writing in the journal Nature, said the effect of a pH drop below 7.8 would be "catastrophic" for the coral.
Coral reefs 'on edge of extinction' - Environment | MSN News - MSN UK