SSDD
Gold Member
- Nov 6, 2012
- 16,672
- 1,966
- 280
Ah, yes. Resorting to ad hominem attack when you know you are wrong.
You resort to reinventing physics when you it doesn't fit with your own preconceived idea.
Here is the scientific definition again.
Spontaneous emission is the process in which a quantum mechanical system (such as an atom, molecule or subatomic particle) transitions from an excited energy state to a lower energy state (e.g., its ground state) and emits a quantum in the form of a photon.
For example, ... there are different forms of luminescence ... fluorescence ... phosphorescence...
I can only suppose it became clear to you that the term spontaneous process and anything associated with it was not going to work for you, so now you have latched on to spontaneous emission a if that were going to work any better.
It is clear that you have no scientific background and little, if any scientific education. You are a poser who is handy with language but who possesses no real depth of understanding of that language.
There is a reason that the second law says that energy can't move spontaneously from a low energy object to a higher energy object...it can't happen via spontaneous process and it can't happen via spontaneous emission. I suppose you don't realize that spontaneous emission is nothing more than a more targeted definition os spontaneous process.
This is where your lack of any real knowledge on the topic becomes glaringly obvious and the fact that you are just grasping at straws...any straw is highlighted in stark relief.
If you are taking about spontaneous processes, the flip side is non spontaneous processes. If you want to talk about spontaneous emission, then the flip side is stimulated emission. Clearly you didn't even bother to look and see if there was a flip side...that whole abject lack of knowledge thing standing in your way and all.
Had you bothered to look, you would have learned that phosphorescence, fluorescence, and every form of luminescence (a very vague term) you might have learned that these are stimulated emissions...not spontaneous emissions. You latched on to the term spontaneous emission with no understanding of what it actually means.
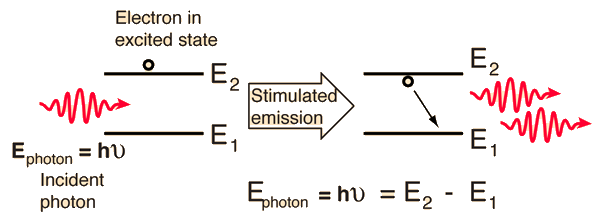
Stimulated emission is the process by which an incoming photon of a specific frequency can interact with an excited atomic electron (or other excited molecular state), causing it to drop to a lower energy level. The liberated energy transfers to the electromagnetic field, creating a new photon with a phase, frequency, polarization, and direction of travel that are all identical to the photons of the incident wave.
Sounds a lot like spontaneous emission except that spontaneous emissionT occurs at random intervals without regard to the ambient electromagnetic field.
Flourescence, of the types of luminescence you noted is the one that is most obviously a stimulated emission. fluorescent materials produce light instantly when atoms inside them absorb energy from a passing EM field (like an electric current), become excited, and return to normal, emitting a photon in the process, all in a tiny fraction of a second.
Fluorescent materials require an outside electrical field...usually a simple electric current. Ergo...stimulated emission...not spontaneous emission. Cut the current and the light goes off shortly thereafter.
Then there are the fluorescent materials like paint, or ink. They absorb energy from certain frequencies of light, become excited, and emit photons of visible light. Switch off the light source and the light show is over. Stimulated emission, stimulated by the external light source.
Phosphorescent materials behave in much the same way as fluorescent materials, but there is usually a longer delay between the time they absorb energy and the time they give off light. Sometimes the phosphorescent materials give off light for fractions of a second and sometimes they give off light for hours, as is the case with your radium watch markers...you have to shine a light on them in order to make them glow...given time, the become dimmer and dimmer till they are no longer visible and require an external light source in order to "recharge". Stimulated emission.
Here are some other types of luminescence so that we don't have to go through the tedious process of you grabbing for every straw in the whole pile.
Bioluminescence - This is made by living creatures...fireflies, glow worms, various marine creatures. The energy to fuel the luminescence is derived from the food they eat. Kill the creature and eventually the luminescence fades and goes out. Stimulated emission.
Chemoluminescence - The energy that provides the light is the result of a chemical reaction. Glow sticks. Crack the glass, shake it up and you get light. But as the chemical reaction runs its course, the energy is used up and the light stick inevitably goes out. Stimulated emission.
Electroluminescence - Already covered...flourescent lights, neon lights etc, Stimulated emission.
Photoluminescence - Already covered....paints, inks, etc. Stimulated emission.
Rontgenoluminesence - The energy that provides the light is X-rays. Turn of the X-ray and the light show is over. Stimulated emission.
Sonoluminescence - In this instance, the energy that produces the light is provided by energetic sound waves usually passed through liquids.
Thermoluminescence - Photons emitted from hot materials...the energy is provided by heat which is usually the result of physical processes. Stimulated emission
Triboluminescence - This sort of luminescence is the result of scratching, rubbing, or physically deforming certain sorts of crystals. The energy is the result of the work expended in rubbing, scratching, or deforming the crystals. Stimulated emission.
If you want to talk about science you must use the scientific definitions.
Here is a clue for you. Knowing the definition isn't enough if you want to talk about science...you also need to have some idea of what that definition means. If that were the case, then we wouldn't have to go through this whole process of you grabbing at every available straw....or in the case of toddster...the only straw he seems to have available..the corona of the sun.
If you understood, even rudimentarily what the definitions mean, then you could apply them to your arguments themselves and see that they fail. I would wager that you were completely ignorant of the term stimulated emission. I mean, if you knew the term, and had even the least inkling of what it meant, you should have been able to grasp that practically any form of luminescence you care to name is the result of stimulated emission.
If you did know, and proceeded with your argument anyway, that would make you a bald faced liar who is perfectly willing to say anything for no other purpose than to trick someone into joining you in your lie. Is that a better description of what you are up to?
The above definition says nothing about what preceded the observation of luminescence.
You are exhibiting an abject failure to understand. You have no idea what you are talking about. You see a thing that will help you and you run with it with no understanding of what you are running with. In this case, and the case of that idiot flashlight example you are running with scissors.
Here is the deal. Take heed if you have a lick of intelligence. There is a reason that spontaneous energy movement from low energy objects to high energy objects resides within the halls of large number statistics. The idea is that if enough of X happens, then some small portion is bound to be Y. True, we never saw it, and can't measure it, but it is bound to happen...so sayeth the statistics of large numbers.
It is that belief that will allow an otherwise intelligent human being to state with a straight face that if you have enough monkeys and enough typewriters, and enough time, they will eventually produce the works of shakespeare. News flash...it isn't going to happen. You only need one typewriter...Add monkeys and time and eventually they might evolve into something that could be capable of producing the works of shakespeare, but then they won't be monkeys any more.
There are no observed, measured examples of energy moving spontaneously from a low energy objects to higher energy objects...there is only the prediction made by the statistics of large numbers. This means that you will never, ever, ever find an example of energy moving spontaneously from low energy to high energy objects. If we could see it, and measure it then the statistics would not be necessary to justify the concept.
Be smart....give it up. Simply acknowledge that what you believe is based on a statistical prediction...probabilities.... and nothing that has ever, nor will ever be observed in reality.
Tearing down all the arguments that you should never have put up in the first place if you were anything like as intelligent as you try to make yourself appear has grown tedious....and the more complex your arguments become, the more obvious it will become that you really don't have a clue.